Topicity
In stereochemistry , topicity (from topography , Greek τόπος tópos , German 'location, location description' ) describes the positional relationship of identical functional groups or atoms (i.e. substituents ) within a molecule relative to one another. Depending on the relationship, such groups can be homotopic , enantiotopic , or diastereotopic . One speaks of homotopicity , enantiotopicity , or diastereotopicity .
Homotopicity
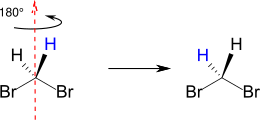
Homotopic groups in chemical compounds are identical groups that have the same chemical environment. Homotopic substituents can be converted into one another by rotating them about a C n axis ( symmetry criterion ). Groups or atoms are referred to as homotopic if the molecule remains achiral when both groups are replaced or if an existing chirality does not change. The substitution does not create a center of chirality (i.e. also no enantiomer, substitution criterion ). Typical examples are the two hydrogen atoms in dibromomethane or the hydrogen atoms in methyl groups. In dibromomethane, for example, the two hydrogen atoms are homotopic. You can swap your position by rotating it by 180 ° along the C 2 axis (red) without changing your surroundings.
Homotopic atoms or groups have an identical chemical shift in nuclear magnetic resonance spectroscopy (NMR) due to their same resonance frequency and are therefore isochronous .
Heterotopicity
If two identical functional groups or atoms are not homotopic, they are referred to as heterotopic. So heterotopic groups have a different environment. These are subdivided into constitutop (different structure or constitution ) or stereoheterotop (different spatial arrangement ). The heterotopicity is composed of enantiotopicity and diastereotopicity.
Enantiotopicity
The stereochemical term enantiotope refers to two identical groups that, if either replaced , would give enantiomers . This creates a center of chirality; therefore these compounds are also referred to as prochiral . Enantiotopic groups can only be converted into one another through a plane of symmetry or an inversion center i , but not through rotation about a C n axis.
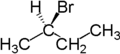
For example, the two hydrogen atoms attached to the second carbon atom of butane are enantiotopic . As a symmetry element, butane has a mirror plane along the carbon chain. If one of these hydrogen atoms is replaced by bromine , ( R ) -2-bromobutane is formed. If the other hydrogen atom is replaced by bromine, the enantiomer is created, i.e. ( S ) -2-bromobutane.
 |
 |

|
| butane | ( R ) -2-bromobutane | ( S ) -2-bromobutane |
Enantiotopic atoms or groups also have the same chemical shift in nuclear magnetic resonance spectroscopy. (As long as the measurement is carried out in a non-chiral environment).
Diastereotopicity
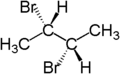
The stereochemical term diastereotopic refers to the position of two groups which, if one of them is replaced ( substituted ), result in diastereomers . For example, the two hydrogen atoms on the third carbon atom of ( S ) -2-bromobutane are diastereotopic. If one of the hydrogen atoms (shown in blue in the picture) is replaced by bromine, a diastereomer ( 2S, 3R ) -2,3-dibromobutane is formed. If the other hydrogen atom (shown in red in the picture) is replaced by bromine, the other diastereomer is formed, ( 2S, 3S ) -2,3-dibromobutane.
 |
 |
|
| ( S ) -2-bromobutane | ( 2S, 3R ) -2,3-dibromobutane | ( 2S, 3S ) -2,3-dibromobutane |
Diastereotopic atoms or groups can show different shifts in nuclear magnetic resonance spectroscopy (NMR), depending on how strong the influence of the chiral environment is on the two atoms or groups. The distance between the diastereotopic groups and the chiral center and the conformational flexibility of the molecule are decisive . Diastereotopic atoms in relatively conformationally stable molecules with several nearby stereocenters often show strikingly large differences in chemical shifts. Typical examples of this are the CH 2 group in carbohydrates (position 6 in hexoses ) and the side chain protons, above all the β protons (HB), the amino acids of folded proteins .
See also
Individual evidence
- ^ Karl-Heinz Hellwich: Stereochemistry - Basic Concepts, Springer Verlag, 2002, pp. 84–87, ISBN 3-540-42347-8 .
- ↑ Eberhard wide Maier, Günther Jung: Organic Chemistry, 7th edition, Thieme Verlag, 2012, pp 283-284, ISBN 978-3-13-541507-9 .
- ^ A b Siegfried Hauptmann, Gerhard Mann: Stereochemie, Spektrum Akademischer Verlag, 1996, pp. 100-103, ISBN 3-86025-144-9 .
- ↑ a b c Joachim Buddrus, Bernd Schmidt: Fundamentals of Organic Chemistry , 5th Edition, de Gruyter Verlag, Berlin, 2015, p. 137, ISBN 978-3-11-030559-3 .