Threonine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
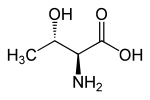
| Structural formula of L- threonine, the naturally occurring isomer | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Threonine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 9 NO 3 | |||||||||||||||||||||
| Brief description |
colorless solid with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 119.12 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
255 ° C ( decomposition , L- threonine) |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Threonine , abbreviated Thr or T , is an essential proteinogenic α - amino acid in its natural L -form .
In threonine there is a hydroxyl group on the β-carbon atom (= 3-position) ; it can also be viewed as 3-methyl- serine or 3-hydroxylated desmethyl- valine . Because of the hydroxyl group, threonine is much more polar and reactive than valine.
Threonine is one of the polar amino acids. It can be phosphorylated on its hydroxyl group, which can play a role in the regulation of enzymes.
Stereochemistry
Threonine has two stereogenic centers on the carbon atoms in the 2- and 3-position. Therefore there are four stereoisomers of threonine with the following absolute configurations: (2 S , 3 R ), (2 R , 3 S ), (2 S , 3 S ) and (2 R , 3 R ). The bound proteins contained in the L threonine has (2 S , 3 R ) configuration and is also provided (2 S , 3 R amino-3-hydroxy-butanoic acid, -2 (designation according to the) IUPAC - Nomenclature ) mentioned. The other three stereoisomers [(2 R , 3 S ) -threonine, (2 S , 3 S ) -allo- threonine and (2 R , 3 R ) -allo- threonine] of L- threonine are of little importance.
When “threonine” is spoken of without any additional name ( prefix ), L- threonine is generally meant.
| Isomers of threonine | ||||
| Surname | L -Threonine | D -Threonine | L - allo- threonine | D - allo- threonine |
| other names | (2 S , 3 R ) -2-amino-3-hydroxybutanoic acid | (2 R , 3 S ) -2-amino-3-hydroxybutanoic acid | (2 S , 3 S ) - allo -2-amino-3-hydroxybutanoic acid | (2 R , 3 R ) - allo -2-amino-3-hydroxybutanoic acid |
| Structural formula |  |
 |
 |

|
| CAS number | 72-19-5 | 632-20-2 | 28954-12-3 | 24830-94-2 |
| 80-68-2 (unspec.) | ||||
| EC number | 200-774-1 | 211-171-8 | 249-327-2 | 246-488-0 |
| 201-300-6 (unspec.) | ||||
| ECHA info card | 100,000,704 | 100.010.157 | 100,044,829 | 100.042.247 |
| 100.001.183 (unspec.) | ||||
| PubChem | 6288 | 69435 | 99289 | 90624 |
| 205 (unspec.) | ||||
| DrugBank | DB00156 | DB03700 | - | - |
| - (unspec.) | ||||
| Wikidata | Q186521 | Q44073885 | Q27103859 | Q27094610 |
| Q60662943 (unspec.) | ||||
Occurrence
Threonine is a component of animal and vegetable proteins . The daily requirement for adults is assumed to be around 16 mg per kg of body weight. The following examples for the threonine content each relate to 100 g of the food; the percentage of the total protein is also given.
| Food | protein | Threonine | proportion of |
|---|---|---|---|
| Beef, raw | 21.26 g | 849 mg | 4.0% |
| Chicken breast fillet, raw | 23.09 g | 975 mg | 4.2% |
| Salmon, raw | 20.42 g | 860 mg | 4.2% |
| Chicken egg | 12.58 g | 556 mg | 4.4% |
| Cow's milk, 3.7% fat | 3.28 g | 148 mg | 4.5% |
| Walnuts | 15.23 g | 596 mg | 3.9% |
| Wholemeal wheat flour | 13.70 g | 395 mg | 2.9% |
| Wholemeal corn flour | 6.93 g | 261 mg | 3.8% |
| Rice, unpeeled | 7.94 g | 291 mg | 3.7% |
| Peas, dried | 24.55 g | 872 mg | 3.6% |
All of these foods contain chemically bound L- threonine as a protein component, only in exceptional cases free L- threonine. In fish, the anti-frost proteins are composed almost exclusively of L- threonine and L- alanine .
history
During his scientific career, the American biochemist William Cumming Rose dealt intensively with the importance of amino acids for nutrition. In experiments on rats in the 1930s, he found that feeding the 19 amino acids known up to then was not sufficient for the rats to grow. He then looked systematically for another essential amino acid; eventually he was able to isolate them from fibrin and identify them according to their structure. With this amino acid known as threonine, the last of the canonical proteinogenic amino acids was discovered. The name threonine was chosen because of the threose basic structure of this amino acid.
properties
The data given here refer only to L- threonine and D- threonine.
- Residual name: Threonyl
- Side chain : hydrophilic
- isoelectric point : 5.64
- Van der Waals volume : 93
- Lipid solubility : log K OW = −0.7
biosynthesis
Since L- threonine is one of the essential amino acids, L- threonine must be taken in with the diet through proteins containing L- threonine. In plants and microorganisms, the biosynthesis of L- threonine begins from L - aspartate , whose origins ( oxaloacetate ) come from the citrate cycle . The L -aspartate is via two intermediate stages, by means of corresponding enzymes (aspartate kinase, aspartate semialdehyde dehydrogenase, homoserine dehydrogenase) for L - homoserine implemented. In a further step, the primary alcohol is phosphorylated by L- homoserine by a homoserine kinase. In the last step, this phosphohomoserine is converted by homoserine phosphate mutaphosphatase (PLP) into L -threonine .
Dismantling
For breakdown, including structural formulas, see section Web links
L threonine is either to acetaldehyde and glycine reduced, which is of the threonine aldolase ( EC 4.1.2.5 catalyzed). The amino acid can also be converted to propionyl-CoA .
Manufacturing
L- threonine can be obtained from protein hydrolysates using the extraction method with the help of ion exchangers . Today, however , L- threonine is mainly produced by fermentation .
use
As a component of amino acid infusion solutions [Aminoplasmal ® (D), Aminosteril ® -N-Hepa (D), Primene ® (A)] for parenteral nutrition, L- threonine, along with other amino acids, is widely used in human medicine. An orally administered “chemically defined diet” containing L- threonine has been developed for patients with impaired digestion . In this diet, the amino acids are the source of nitrogen; all vital nutrients are in a chemically precisely defined form.
Many types of grain have too low an essential amino acid content. As a result of this lack of just one amino acid, the usability of all the amino acids taken in drops to the value determined by the essential amino acid ("limiting amino acid") contained in too small a quantity. The nutritional value of the grain is then increased by the targeted addition of small amounts of those essential amino acids that are deficient in it. With the exception of maize, most cereals contain less L- threonine than is required by livestock. The addition of L- threonine to compound feed is widespread in the feed industry and thus conserves natural resources.
Web links
Individual evidence
- ↑ a b c data sheet threonine (PDF) from Merck , accessed on December 25, 2019.
- ↑ a b Data sheet L-Threonine, 98 +% at AlfaAesar, accessed on December 25, 2019 ( PDF )(JavaScript required) .
- ^ A b c Carl S. Vestling, Donald T. Warner: The isoelectric points of threonine and some related compounds . In: Journal of Biological Chemistry . tape 144 , no. 3 , 1942, pp. 687–690 (English, jbc.org [PDF]).
- ↑ Entry on L-threonine. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ Threonine data sheet from Sigma-Aldrich , accessed on October 17, 2016 ( PDF ).
- ↑ DH Baker: Tolerance for branched-chain amino acids in experimental animals and humans , in: J. Nutr. , 2005 , 135 (6) , pp. 1585S-1590S; PMID 15930474 .
- ↑ U.S. Department of Agriculture Nutrient Database , 21st Edition.
- ^ GC Barrett: Chemistry and Biochemistry of the Amino Acids , Chapman and Hall, London, New York, 1985, p. 11, ISBN 0-412-23410-6 .
- ↑ S. Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois. ( Memento from June 15, 2016 in the Internet Archive ) Berlin 2015.
- ↑ WC Rose, Feeding experiments with mixtures of highly purified amino acids: I. The inadequacy of diets containing nineteen amino acids , J Biol Chem, Volume 94, p. 155ff (1931)
- ↑ WC Rose, RH McCoy and CE Meyer, Feeding experiments with mixtures of highly purified amino acides: VIII. Isolation and identification of a new essential amino acid , J Biol Chem, Volume 112, p. 283ff. (1935).
- ↑ Bernd Hoppe and Jürgen Martens: Amino acids - production and extraction , in: Chemistry in our time , 1984 , 18 , pp. 73–86; doi: 10.1002 / ciuz.19840180302 .
- ↑ a b Yoshiharu Izumi, Ichiro Chibata, Tamio Itoh: Production and Use of Amino Acids , in: Angewandte Chemie (journal) , 1978, 90 (3), pp. 187-194; doi: 10.1002 / anie.19780900307 .