Steroid alkaloids
The steroid alkaloids are a group of pseudo alkaloids . They are nitrogen-containing steroids. The steroid alkaloids include the Solanum , Veratrum, Apocynacean steroid alkaloids, Buxus steroid alkaloids, as well as the salamander alkaloids and the batrachotoxins.
Examples
Batrachotoxins
| structure | Natural occurrence |
|
|
Batrachotoxins form on the skin of South American poison dart frogs . The photo shows the Phyllobates terribilis ('terrible poison dart frog'). |
The neurotoxic batrachotoxins can be found naturally on the skin of poison dart frogs . They are based on the structure of the pregnanes, which have 21 carbon atoms. The amino group on the 18th carbon atom is specific for the batrachotoxins. An example of this is the batrachotoxin A.
Bufotoxins
| structure | Natural occurrence |
|
|
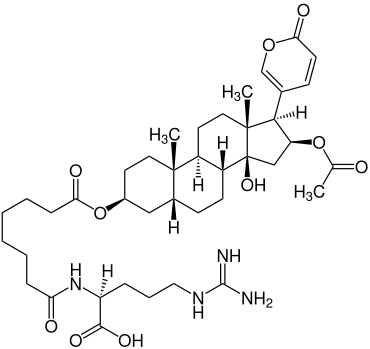
Bufotoxin is produced on the skin of toads of the genus real toads ( Bufo ). |
The bufotoxins are named after the genus of bufo . The α-pyranones on the 17th carbon atom are specific for the bufotoxins. The bufotoxin shown here is a sterane derivative with an α-pyranone on the 17th carbon atom and an esterified suberic acid on the 3rd carbon atom with L-arginine attached to it .
Buxus steroid alkaloids
| structure | Natural occurrence |
|
|
Buxamin E, buxandolin L and cyclobuxin D are found in the leaves of common boxwood (buxus sempervirens). |
The Buxus steroid alkaloids are found in the leaves of the common boxwood ( Buxus sempervirens ). This plant is found mainly in southern and central Europe. The Buxus steroid alkaloids have an amino group attached to the 3rd and / or 20th carbon. The amino groups are partially, completely or not at all methylated. The Buxus steroid alkaloids are a large group of bases, most of which can be divided into three groups.
Another group of Buxus steroid alkaloids has a structure with a tetracyclic system. These have a bond between the 9th and 19th carbon atoms, so that ring B is a seven-membered ring. A representative of this group is the buxamin E.
The third large group is characterized by the fact that no other carbon atoms are bonded to the 4th carbon atom of the A-ring. An example of this is the buxandonin L.
The largest group are the box steroid alkaloids, which are pentacyclic. They are based on the structure of 4,4,14-trimethyl-9,19-cyclopregnane. One representative of this group is cyclobuxin D.
Apocynacean steroid alkaloids
| structure | Natural occurrence |
|
|
Latifolinin is found in the bark of Funtumia latifolia . The leaves of this plant also contain funtumin and funtumidin. These are apocynacean steroid alkaloids with the 5α-pregnane backbone. |
The Apocynacean steroid alkaloids can be based on the 5α-pregnan, Δ 5 -pregnen or the conanine skeleton. These usually have an amino group or an oxygen compound on the 3rd carbon atom. An example of this is latifolinin. The latifolinin can be derived from the conanin skeleton. A five-membered ring, formed by an amino group attached to both the 18th and 20th carbon, is characteristic of this.
Salamander alkaloids
| structure | Natural occurrence |
|
|
Salamander alkaloids such as B. Samandarin occur on the skin of animals of the genus Salamandra. |
The toxic salamander alkaloids can of course be found in the organisms of animals belonging to the genus Salamandra. They can be derived from the 3- aza- A -homo-5β-androstane. One example is Samandarin (see illustration), which, depending on the breed, can be the main alkaloid, but can also not be part of the organism at all. The oxazolidine structure on the A ring is specific to samandarin. In addition to Samandarin there are some other steroid alkaloids in the organisms of Salamandra such as B. Samandaridin, Samandaron and Cycloneosamandione.
Solanum alkaloids
Solanidan skeleton
| structure | Natural occurrence |
|
|
Solanocapsin comes u. a. in the fruits of the coral bush ( Solanum pseudocapsicum ). |
The steroid alkaloids with a solanidane skeleton are characterized by a bicyclic system that replaces a cholesterol side chain on the D-ring. An example of this is the solanocapsin found in the coral bush ( Solanum pseudocapsicum ).
Spriosolan basic structure
| structure | Natural occurrence |
|
|
Tomatidenol comes u. a. in the leaves of the bitter-sweet nightshade ( Solanum dulcamara ). This species of plant belongs to the nightshade genus. Tomatidenol forms the main alkamin in the species of Solanum dulcarama , which are native to Europe. |
Another type of Solanum alkaloids is based on the Spirosolan framework. In these, the E-ring is a tetrahydrofuran to which a piperidine is bound directly via a spiro compound. An example of such a steroid alkaloid is Tomatidenol. This occurs in many species of the genus Solanum .
Veratrum alkaloids
Veratrum alkaloids of the white / green Germers
| structure | Natural occurrence |
|
|
Procevin and Veratramin come u. a. in the white Germer ( Veratrum album grandiflorum ). |
The Veratrum alkaloids are named after the white or green Germer ( Veratrum album or Veratrum viride ). These plants belong to the Liliaceae family. Procevin is a special case in these, as the nitrogen of the piperidine is connected to the 18th carbon.
Veratramine is an example of Veratrum steroid alkaloids, which are based on a 22,26-epimino-14- abeo- cholestane ring system.
Veratrum alkaloids of the Sabadill
| structure | Natural occurrence |
|
|
Veracevin occurs in the Sabadill ( Schoenocaulon officinale ). |
Veracevin is also one of the Veratrum alkaloids. However, this occurs in the Sabadill ( Schoenocaulon officinale ), which also belongs to the Liliaceae family. The Veracevin is based on the Cevan framework, in which the C-ring is a five- instead of a six-membered ring and the D-ring is a six-membered ring. The high number of hydroxyl groups is also noteworthy.
Individual evidence
- ↑ Entry on steroid alkaloids. In: Römpp Online . Georg Thieme Verlag, accessed on February 25, 2019.
- ^ Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. 86 .
- ↑ a b Wolfgang Bücherl; Eleanor E. Buckley (Ed.): Venomous Animals and Their Venoms . tape 2 . Academid Press, New York 1971, ISBN 978-0-12-138902-4 , pp. 261 , doi : 10.1016 / C2013-0-10436-9 .
- ↑ a b c Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. XXV-XXVI .
- ↑ Yoshiaki Kamano; Hiroshi Yamamoto; Yoshihiro Tanaka; Manki Komatsu: The Isolation and Structure of new Bufadienolides, 3- (Hydrogen suberates) of Resibufogenin, Cinobufagin and Bufalin - the Structure of the so-called "Bufotoxins" . In: Tetrahedron Letters . tape 9 , no. 54 , 1968, pp. 5673-5676 , doi : 10.1016 / S0040-4039 (00) 70748-9 .
- ↑ Kazutake Shimada; Kazuo Ohishi; Hiroko Fukunaga; Jai Seup Ro; Toshio Nambara: Structure-activity relationship of bufotoxins and related compounds for the inhibition of Na +, K + -adenosine triphosphatase . In: Journal of Pharmacobio-Dynamics . tape 8 , no. 12 , 1985, pp. 1054-1059 , doi : 10.1248 / bpb1978.8.1054 .
- ↑ Wolfgang Bücherl; Eleanor E. Buckley (Ed.): Venomous Animals and Their Venoms . tape 2 . Academid Press, New York 1971, ISBN 978-0-12-138902-4 , pp. 546 , doi : 10.1016 / C2013-0-10436-9 .
- ↑ a b O. Gessner, G. Barger: Handbook of experimental pharmacology . Ed .: W. Heubner, J. Schüller. tape 6 . Springer-Verlag Berlin Heidelberg, Berlin 1938, ISBN 978-3-662-32094-5 , pp. 45 , doi : 10.1007 / 978-3-662-32921-4 .
- ↑ a b Eberhard Breitmaier: Alkaloids: narcotics, hallucinogens and other active ingredients, lead structures from nature . 3. Edition. Vieweg + Teubner - GWV Fachverlage GmbH, Wiesbaden 2008, ISBN 978-3-8348-0531-7 , p. 97-98 .
- ↑ a b P. H. List, L. Hörhammer (Ed.): Hager's Handbook of Pharmaceutical Practice: Chemicals and Drugs (Am-Ch) . 5th edition. tape 3 . Springer-Verlag Berlin Heidelberg GmbH, Berlin 1972, ISBN 978-3-642-80563-9 , p. 589-590 , doi : 10.1007 / 978-3-642-80562-2 .
- ^ Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. 644 .
- ↑ a b R. Hegnauer: Chemotaxonomy of plants: An overview of the distribution and the systematic importance of plant substances . tape 3 . Springer Basel AG, Basel 1964, ISBN 978-3-0348-9386-2 , p. 127-129 , doi : 10.1007 / 978-3-0348-9385-5 .
- ^ Apocynacean steroid alkaloids . In: Burkhard Fugmann (Ed.): Römpp Lexikon Naturstoffe . 10th edition. tape 1 . Georg Thieme Verlag, Stuttgart 1997, ISBN 978-3-13-199961-0 , p. 50 .
- ^ Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. 769 .
- ↑ T. Lüddecke; S. Schulz; S. Steinfartz: A salamander's toxic arsenal: review of skin poison diversity and function in true salamanders, genus Salamandra . In: The Science of Nature . tape 105 , no. 56 . Springer Berlin Heidelberg, 2018, p. 208-216 , doi : 10.1007 / s00114-018-1579-4 .
- ↑ Entry on salamander alkaloids. In: Römpp Online . Georg Thieme Verlag, accessed on February 24, 2019.
- ↑ Atta-ur-Rahman (Ed.): Studies in Natural Products in Chemistry: Structure and Chemistry (Part C) . tape 15 . Elsevier, Amsterdam 1995, ISBN 0-444-82083-3 , pp. 337 .
- ^ Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. 801 .
- ↑ a b Eberhard Breitmaier: Alkaloids: narcotics, hallucinogens and other active ingredients, lead structures from nature . 3. Edition. Vieweg + Teubner - GWV Fachverlage GmbH, Wiesbaden 2008, ISBN 978-3-8348-0531-7 , p. 90 .
- ^ Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. 858 .
- ↑ R. Hegnauer: Chemotaxonomy of plants: An overview of the distribution and the systematic importance of plant substances . tape 7 . Springer Basel AG, Basel 1986, ISBN 978-3-0348-9991-8 , p. 427 , doi : 10.1007 / 978-3-0348-9314-5 .
- ↑ Helmut Ripperger Klaus Schreiber: Solanum Alkaloide, LXXXIX. Synthesis of the steroid alkaloid leptinidine and other 23β ‐ hydroxy ‐ solanidane . In: Chemical Reports . tape 102 , no. 12 . WILEY ‐ VCH Verlag GmbH & Co., Weinheim 1969, p. 4080-4088 , doi : 10.1002 / cber.19691021215 .
- ^ Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. 759 .
- ^ Dictionary of steroids . Chapman & Hall, London 1991, ISBN 0-412-27060-9 , pp. 943 .
- ↑ R. Hegnauer: Chemotaxonomy of plants: An overview of the distribution and the systematic importance of plant substances . tape 7 . Springer Basel AG, Basel 1986, ISBN 978-3-0348-9991-8 , p. 711 , doi : 10.1007 / 978-3-0348-9314-5 .
- ↑ a b Jakob Büchi: Fundamentals of drug research and synthetic drugs . Springer Basel AG, Basel 1963, ISBN 978-3-0348-4020-0 , p. 45-46 , doi : 10.1007 / 978-3-0348-4019-4 .
- ↑ a b c Veratrum steroid alkaloids . In: Burkhard Fugmann (Ed.): Römpp Lexikon Naturstoffe . 10th edition. Georg Thieme Verlag, Stuttgart 1997, ISBN 978-3-13-200061-2 , p. 680-681 .
- ↑ Friedrich Constabel; Indra K. Vasil (Ed.): Cell Culture and Somatic Cell Genetics of Plants: Phytochemicals in Plant Cell Cultures . tape 5 . Academic Press, Inc., San Diego 1984, ISBN 0-12-715005-6 , pp. 538 .