Pseudoalkaloids
In contrast to that of the alkaloids, the carbon structure of the pseudoalkaloids does not come from amino acids , but is mostly formed from isoprenoid compounds or polyketides . The nitrogen atom is usually incorporated in the course of the biosynthesis in a later phase in the form of NH 3 . The peptide alkaloids also belong to the group of pseudoalkaloids.
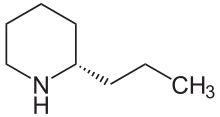
Albert Ladenburg achieved the first synthesis of a pseudoalkaloid in 1886 with the synthesis of coniin .
In the biosynthesis of coniin, four acetates react formally with ammonia via a hypothetical polyketide intermediate to form γ-conicein (a cyclic imine ). Coniin provides its subsequent reduction.
literature
- Ernesto Fattorusso: Modern Alkaloids: Structure, Isolation, Synthesis and Biology , 689 pages, Wiley-VCH Verlag GmbH & Co. KGaA (October 24, 2007), ISBN 3-527-31521-7 .
Individual evidence
- ^ Peter Nuhn : Naturstoffchemie 2nd edition, S. Hirzel Wissenschaftliche Verlagsgesellschaft Stuttgart, p. 553, ISBN 3-7776-0473-9 .