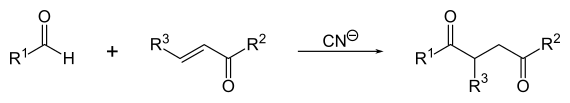
Stetter reaction
The Stetter reaction is a chemical reaction that is used to synthesize 1,4- diketones . An aldehyde and a Michael acceptor (α, β-unsaturated ketone , see also Michael addition ) are used as starting materials for this cyanide- catalyzed reaction . Α, β-unsaturated nitriles or esters can also be reacted. The reaction is named after the German chemist Hermann Stetter .
Reaction mechanism
First, the cyanide ion reversibly adds to the aldehyde. The alcoholate formed tautomerizes to a carbanion , which then attacks the Michael acceptor in a nucleophilic manner . The desired diketone is obtained by splitting off the catalytically added cyanide and subsequent keto-enol tautomerism .
variants
Instead of cyanide ions, ylides produced from thiazolium salts can also be used.
If α, β-unsaturated nitriles are used as acceptors, then γ-ketonitriles can be synthesized by the Stetter reaction.
Individual evidence
- ^ Hermann Stetter: The catalyzed addition of aldehydes to activated double bonds - A new synthetic principle , Angewandte Chemie 1976 , 88 , 695-736.
literature
Hans Beyer , Wolfgang Walter : Textbook of Organic Chemistry ; S. Hirzel Verlag, Stuttgart - Leipzig 1998, 23rd revised. and updated edition; ISBN 3-7776-0808-4 .