Adiponitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
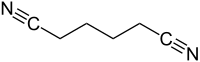
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Adiponitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 8 N 2 | |||||||||||||||
| Brief description |
colorless, almost odorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 108.14 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.96 g cm −3 |
|||||||||||||||
| Melting point |
2.3 ° C |
|||||||||||||||
| boiling point |
295 ° C |
|||||||||||||||
| Vapor pressure |
0.3 Pa (20 ° C) |
|||||||||||||||
| solubility |
soluble in water (50 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4380 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Adiponitrile (ADN, Adipinsäuredinitril, Hexandisäuredinitril) belongs to the aliphatic nitriles and is an intermediate product of the polyamide production .
Manufacturing
Adiponitrile is mainly produced by the hydrocyanation of 1,3-butadiene . In this case, hydrogen cyanide using a nickel catalyst to the double bonds is added the butadiene.
Two other processes are based on the dimerization of acrylonitrile .
- The electrochemical reduction leads directly to adiponitrile via hydrodimerization. The cell for electrosynthesis contains anodically diluted sulfuric acid and cathodically separated via a diaphragm an aqueous solution of acrylonitrile and salts of 4-toluenesulfonic acid as a solubilizer for the slightly water-soluble acrylonitrile.
- The catalytic dimerization gives unsaturated hexenedinitrile, which is hydrogenated to adiponitrile .
In the laboratory, it can be produced by nucleophilic substitution of 1,4-dichlorobutane with NaCN in DMSO .
A biotechnological process for the production of adiponitrile was recently reported, in which the use of toxic hydrogen cyanide (or cyanides) is dispensed with. The key step is the double elimination of water from the hexanedialdoxime, during which adiponitrile is formed. This step is catalyzed by an aldoxime dehydratase.
use
Adiponitrile is extensively hydrogenated to hexamethylenediamine . Adipic acid can be produced by hydrolysis of the nitrile groups. Both products are required for the manufacture of PA6.6 .
Individual evidence
- ↑ a b c d e f g h i Entry on adiponitrile in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-10.
- ↑ Wolfgang-Dieter Luz, Eberhard Zirngiebl: The future of electrochemistry. In: Chemistry in Our Time. 23, 1989, pp. 151-160, doi : 10.1002 / ciuz.19890230503
- ↑ Synthesis of adiponitrile - PrepChem.com. Accessed July 11, 2018 .
- ↑ Harald Gröger : Biocatalysis - Solution approaches along the chemical value chain BIOspektrum 25 (2019), pp. 786–789, doi: 10.1007 / s12268-019-0093-3 .
- ↑ T. Betke, M. Maier, H. Gruber-Wölfler, H. Gröger: Biocatalytic production of adiponitrile and related aliphatic linear α, ω-dinitriles , Nature Commun. 2018, 9, 5112, doi: 10.1038 / s41467-018-0743-0 .


