Negishi clutch
The Negishi coupling is a name reaction from organic chemistry . The cross-coupling is used for the alkenylation or arylation of aryls. For this purpose, aryl halides or triflates are reacted with organozinc compounds in a nickel or palladium catalyzed reaction . The reaction is related to the Stille , Suzuki, and Kumada coupling . The reaction is named after its discoverer, the Japanese chemist Ei-ichi Negishi , who published it in 1977 and was awarded the Nobel Prize for Chemistry in 2010.

Suitable ligands are phosphines such as triphenylphosphine or dppe and the chiral chelate ligands such as BINAP or Chiraphos .
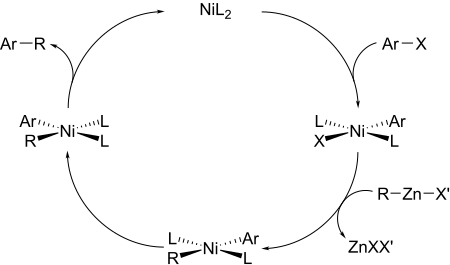
Reaction mechanism
In the first step , the aryl halide used adds oxidatively to the nickel or palladium catalyst . The oxidation state of the metal changes from 0 to +2. The second step of the cycle consists of a transmetallation , in which the remainder of the zinc to be coupled is transmetaled onto the catalyst metal. After possible isomerization of trans - to cis - complex the desired is product is reductively eliminated . Here the catalyst species is regressed.
scope of application
The Negishi coupling is widely used because it tolerates many functional groups . This is due to the relative inertness of the organozinc compounds compared to other organometallic organyls that can be used in coupling reactions.
The zinc organyls required can usually be obtained from the corresponding organolithium compound .
- Transmetallation from lithium to zinc using zinc bromide . Lithium bromide is produced as a by-product .
Individual evidence
- ↑ a b Reinhard Brückner: reaction mechanisms . 3rd edition, pp. 706–708, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 .
- ↑ AO King, N. Okukado, E.-i. Negishi: Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the Pd-catalysed reaction of alkynylzinc reagents with alkenyl halides , in: Chem. Comm. 1977 , 683; doi: 10.1039 / C39770000683 .