Hentriacontanoic acid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
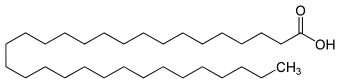
|
||||||||||
| General | ||||||||||
| Surname | Hentriacontanoic acid | |||||||||
| Molecular formula | C 31 H 62 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 466.83 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
93.1 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Hentriacontanoic acid is a long-chain, saturated fatty acid with an odd number of carbon atoms. The alkanoic acid belongs to the group of wax acids .
The fatty acid occurs in leaf wax; for example, it was isolated from the sisal agave ( Agave sisalana ). In the past it was often confused with melissic acid .
Individual evidence
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 93rd edition. (Internet version: 2012), CRC Press / Taylor and Francis, Boca Raton, FL, Biochemistry, pp. 7-8.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ J. Buckingham: Dictionary of Organic Compounds. Vol. 4: F-Mer , Sixth Edition, Chapman & Hall, 1996, ISBN 0-412-54090-8 , p. 3391.
- ↑ Entry on hentriacontanoic acid. In: Römpp Online . Georg Thieme Verlag, accessed on November 5, 2017.
- ^ Jürgen Falbe, Manfred Regitz: RÖMPP Lexikon Chemie. Volume 6: T – Z , 10th edition, Thieme, 1999, ISBN 3-13-735110-3 , p. 4630.