Von Baeyer nomenclature
The Von Baeyer nomenclature is a nomenclature system for saturated, bridged polycyclic hydrocarbons . The system was originally developed in 1900 by Adolf von Baeyer for bicyclic systems and expanded in 1913 by Eduard Buchner and Wilhelm Weigand for tricyclic systems. The rules were later extended by the IUPAC to other classes of compounds such as heterocycles , siloxanes or organosilicon and organoboron compounds .
| Bridged Polycyclic Hydrocarbons (Examples) |
|---|
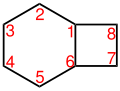 Bicyclo [4.2.0] octane |
 Tricyclo [4.3.0.0 2.9 ] nonane |
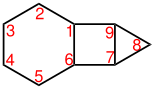 Tricyclo [4.3.0.0 7.9 ] nonane |
designation
Bridged polycyclic hydrocarbons are in accordance with this nomenclature, according to the number of rings as bicyclo -, tricyclo etc. - alkane designated. The ending results from the total number of carbon atoms in the molecule. The number of rings can be determined by the number of bond cleavages that are necessary to achieve an open-chain shape. Between the prefix and the ending, the sizes of the bridges are given in square brackets - in descending numbers - and separated by periods.
Naming
| Heterocyclic Bridged Polycyclic Compound (Example) |
|---|
 1,4-diazabicyclo [2.2.2] octane (DABCO) |
In order to get the systematic name of a compound according to the Von Baeyer system, the number of rings and the size of the main ring must first be determined. For this purpose, a three-dimensional structural formula is expediently represented two-dimensionally. The carbon atoms at which the bridges between parts of the main ring begin are the so-called bridgeheads . The longest bridge (with the most carbon atoms outside the main ring) is called the main bridge , and the associated bridgeheads are called the main bridgeheads . If there are several main bridges of exactly the same length, then the bridge becomes the main bridge, which divides the main ring more evenly (see example Tricyclo [4.3.0.0 7,9 ] nonane).
The numbering of the carbon atoms begins at one of the main bridgeheads. This then first runs along the main ring, then possibly existing atoms of the main bridge and finally those of the other bridges. It is always numbered so that the smallest possible numbers are obtained.
The numbers in square brackets are determined by the number of carbon atoms in the bridges between the main bridgeheads. The main bridgeheads themselves are not counted. The first digit is determined by the length of the longest bridge along the main ring, followed by the number of carbon atoms in the other part of the main ring and the number of atoms in the main bridge.
If there are one or more secondary bridges in addition to the main bridge, their sizes are written in descending order after the number of carbon atoms in the main bridge. In addition, the numbers of the bridgehead carbon atoms are written in superscript behind the number.
Saturated, heterocyclic systems can also be named after this system, such as the systematic name of DABCO , 1,4-diazabicyclo [2.2.2] octane.
Emergence
After numerous hydrocarbons had been discovered in the 19th century, the carbon atoms of which formed two rings, Baeyer felt compelled to propose a systematics and nomenclature for (bicyclic) hydrocarbons which " contain two or more carbon atoms common to both rings ." He referred to these hydrocarbons as "Bicyclic" and stated:
“ Every bicyclic hydrocarbon contains two tertiary carbon atoms that are linked three times either directly or through interposed atoms. These compounds are called bridges, and are denoted by the number of carbon atoms they are made of. The number 0 means the direct connection of the two tertiary atoms, the number 1 the interim storage of an atom, etc ... "
The word “connection” was not used in today's sense by Baeyer, it was only intended to express topological relationships; i.e., in today's language as a graph . “Link lines” symbolized links between the carbon atoms, which were thought of as dots. Because the “nature of the chemical bond” was unknown at the time. (Today we can see that Baeyer's definition had the advantage of not being dependent on the nature of the types of attachment discovered later.)

Baeyer characterized these molecular systems by the number of bridging atoms, which in increasing size, separated by commas and in square brackets, provided the so-called characteristic . For example, should norcarane get heptane name bicyclo [0, 1, 4]. Baeyer's characteristics were later modified as described above. Today, Norcaran bears the name Bicyclo [4.1.0] heptane.
Individual evidence
- ^ A b Adolf Baeyer: Systematics and nomenclature of bicyclic hydrocarbons. In: Reports of the German Chemical Society 1900, 33, pp. 3771-3775, doi : 10.1002 / cber.190003303187 .
- ^ E. Buchner, W. Weigand: Bornylen und Diazoessigester [In addition to a nomenclature of tricyclic carbon ring systems according to Adolf von Baeyer] . In: Ber. German Chem. Ges. 1913, 46, 2108-2117, doi : 10.1002 / cber.191304602130 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 213, ISBN 3-342-00280-8 .
literature
- GP Moss: Extension and revision of the von Baeyer system for naming polycyclic compounds (including bicyclic systems). In: Pure Appl. Chem. 1999, 71, 3, pp. 513-529 ( full text ; PDF; 302 kB). (IUPAC publication, English).
- Karl-Heinz Hellwich: Extension and revision of the von Baeyer system for naming polycyclic compounds (including bicyclic compounds). In: Angew. Chem. 2002, 114, 3423-3432, doi : 10.1002 / 1521-3757 (20020902) 114: 17 <3423 :: AID-ANGE3423> 3.0.CO; 2-6 . (official German translation of the IUPAC publication).
- D. Hellwinkel: The systematic nomenclature of organic chemistry. 4th edition, Springer, Berlin 1998, ISBN 3-540-63221-2 , pp. 29-32.