Quadricyclan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
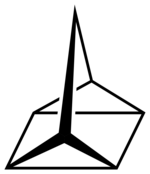
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Quadricyclan | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 8 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 92.14 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.982 g cm −3 |
|||||||||||||||
| Melting point |
−44 ° C |
|||||||||||||||
| boiling point |
108-111 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Quadricyclane is a strained, multicyclic hydrocarbon that is being discussed as a potential candidate for rocket fuels and solar energy storage . These applications are limited by the relatively low decomposition temperature of below 400 ° C.
Structure and properties
Quadricyclane is a highly strained molecule (329.5 kJ / mol). The isomerization to norbornadiene takes place slowly without catalysts at low temperatures. Because of its strained structure and thermal stability, it has been extensively studied.
Manufacturing
Quadricyclan is produced by irradiating norbornadiene with UV radiation of approx. 300 nm in the presence of sensitizers such as Michler's ketone . Other sensitizers such as acetone , copper (I) chloride , benzophenone , acetophenone can also be used, but give poorer yields.
The reverse reaction is possible in the presence of metal complex catalysts such as metal porphyrins at temperatures above 100 ° C.
Storage of solar energy
Through the isomerization of norbornadiene to quadricyclane, an energy of Δ H = −89 kJ / mol is 'stored'. The system can therefore potentially be used as a storage facility for solar energy . Since the main absorption of this reaction is around 300 nm, but only small portions of the sunlight are below 400 nm, this use is limited.
Reactions
Quadricyclane reacts smoothly with acetic acid to form a mixture of nortricyclyl acetate and exo -norbornyl acetate. Similar to cyclopropane, Quadricyclane enters into addition reactions, e.g. B. also with bromine or hydrogen. This results in norbornene or norbornadiene derivatives. It also reacts with many dienophiles to form 1: 1 adducts.
literature
- EJ Wucherer, Angelica Wilson: Chemical, Physical and Hazards Properties of Quadricyclane , Air Force Research Laboratory, Edwards Air Force Base CA 93524-7048, March 1998 ( PDF ).
- Beyer / Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , p. 390.
- Streitwieser / Heathcock : Organic Chemistry , 1st edition, Verlag Chemie, Weinheim 1980, ISBN 3-527-25810-8 , pp. 1351-1352.
Individual evidence
- ↑ a b Claibourne D. Smith: Quadricyclane In: Organic Synthesis . 51, 1971, p. 133, doi : 10.15227 / orgsyn.051.0133 ; Coll. Vol. 6, 1988, p. 962 ( PDF ).
- ↑ a b c Exciton: QUADRICYCLANE Datasheet
- ↑ a b Datasheet Quadricyclane, 99% from Sigma-Aldrich , accessed on March 18, 2014 ( PDF ).
- ↑ a b Petrov, V. A; Vasil'ev, NV “Synthetic Chemistry of Quadricyclane” Current Organic Synthesis (2006) 3, pp. 215-259 ( abstract ).
- ↑ PS Kalsi: Organic Reactions And Their Mechanisms. New Age International, 2000, ISBN 978-8-122-41268-0 , p. 366 ( limited preview in Google book search).
- ↑ Patent application US20040054244 : Process of quadricyclane production. Filed September 12, 2003 , published March 18, 2004 , applicant: Paul Cahill, Richard Steppel, inventor: Exciton.
- ↑ a b Dieter Wöhrle, Michael W. Tausch, Wolf-Dieter Stohrer: Photochemistry: Concepts, Methods, Experiments - Dieter Wöhrle, Michael W. Tausch, Wolf-Dieter Stohrer . John Wiley & Sons, 2012, ISBN 3-527-66088-7 ( limited preview in Google Book Search).
- ↑ Dubonosov, A. D; Bren, V. A; Chernoivanov, VA "Norbornadiene - quadricyclane as an abiotic system for the storage of solar energy." Russ. Chem. Rev. (Engl. Transl.) 71 (2002): pp. 917-927.
- ↑ Constantine Philippopoulos, Dimitrios Economou, Constantine Economou, John Marangozis: Norbornadiene-Quadricyclane System in the Photochemical Conversion and Storage of Solar Energy . In: Ind. Eng. Chem. Res . 22, No. 4, 1983, p. 627. doi : 10.1021 / i300012a021 .
- ↑ Hans-Dieter Jakubke, Ruth Karcher (coordinators): Lexikon der Chemie in three volumes, Spektrum Verlag, Heidelberg, Volume 3, 1999, ISBN 3-8274-0381-2 , p. 131.


