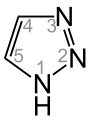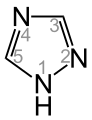Triazoles
As triazoles are heterocyclic aromatic compounds having the formula C 2 H 3 N 3 designates which a pentatomic ring with two carbon and three - nitrogen - atoms contained.
Isomers
Depending on the arrangement of the three nitrogen atoms in the heteroaromatic five-membered ring, there are two isomeric triazoles, each of which occurs in two tautomeric forms (difference: position of the nitrogen atom in the ring to which a hydrogen atom is bound):
- 1,2,3-triazole (exists in two tautomeric forms: 1 H -1,2,3-triazole and 2 H -1,2,3-triazole)
- 1,2,4-triazole (exists in two tautomeric forms: 1 H -1,2,4-triazole and 4 H -1,2,4-triazole)
use
Many derivatives of triazoles are used as drugs ( antifungal agents , including fluconazole , fosfluconazole , itraconazole , voriconazole and posaconazole ) or plant protection agents ( fungicides , such as cyproconazole , difenoconazole , epoxiconazole , flusilazole , hexaconazole , propiconazole , tebuconazole , tetraconazole or triadimenol used).
Triazoles are the active ingredients in many commercially distributed, approved and applied fungicides for controlling plant diseases such as Septoria tritici or Fusarium species. They inhibit the ergosterol biosynthesis in fungi. Some mutations in the gene coding for the target enzyme triazoles have been linked to differences in the sensitivity of the fungi to triazoles. Only triazoles are permitted for combating some plant diseases.
Many newly developed, insensitive yet powerful explosives are derivatives of triazole, such as nitrotriazolone (NTO) or aminonitrotriazole (ANTA).
See also
Individual evidence
- ↑ John A. Joule, Keith Mills: Heterocyclic Chemistry at a Glance. 2nd Edition. Wiley & Sons, ISBN 978-0-470-97121-5 , pp. 132-136.
- ↑ a b List of approved plant protection products.
- ↑ HJ Cools, BA Fraaije, SH Kim, JA Lucas: Impact of changes in the target P450 CYP51 enzyme associated with altered triazole-sensitivity in fungal pathogens of cereal crops. International Symposium on Cytochrome P450. In: Biodiversity and Biothechnology. 8, 2006, pp. 1219-1223, (PDF) .