Xylenes
The xylenes (from ancient Greek ξύλον xýlon " wood "), also xylenes or according to the IUPAC nomenclature dimethylbenzene , are liquid organic-chemical compounds with a characteristic aromatic odor and the general formula C 8 H 10 . They are among the aromatic hydrocarbons and each consist of a benzene ring with two methyl substituents (–CH 3 ). By different arrangements of the methylene groups there are three structural isomers of xylene: 1,2-xylene ( o rtho-xylene), 1,3-xylene ( m eta-xylene) and 1,4-xylene ( p ara-xylene). In technology (e.g. as a solvent) they are mostly used as an (unseparated) mixture of isomers and are usually composed of 60% m -xylene, 10-25% o -xylene and 10-25% p -xylene. Xylene mixtures used as solvents often also contain ethylbenzene , which boils in the same temperature range and has similar dissolving properties.
Representative
| Xylenes | ||||||||||||
| Surname | o -xylene | m -xylene | p -xylene | |||||||||
| other names | 1,2-dimethylbenzene 1,2-dimethylbenzene |
1,3-dimethylbenzene 1,3-dimethylbenzene |
1,4-dimethylbenzene 1,4-dimethylbenzene |
|||||||||
| Structural formula |
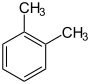
|

|
|
|||||||||
| CAS number | 95-47-6 | 108-38-3 | 106-42-3 | |||||||||
| 1330-20-7 (mixture of isomers) | ||||||||||||
| PubChem | 7237 | 7929 | 7809 | |||||||||
| ECHA InfoCard | 100.002.203 | 100.003.252 | 100.003.088 | |||||||||
| 100.014.124 (mixture of isomers) | ||||||||||||
| Molecular formula | C 8 H 10 | |||||||||||
| Molar mass | 106.17 g mol −1 | |||||||||||
| Physical state | liquid | |||||||||||
| Brief description | colorless liquid | |||||||||||
| Melting point | −25 ° C | −48 ° C | 13 ° C | |||||||||
| boiling point | 144 ° C | 139 ° C | 138 ° C | |||||||||
| Solubility in water | 180 mg l −1 | 174 mg l −1 | 200 mg l −1 | |||||||||
|
GHS labeling |
from Regulation (EC) No. 1272/2008 (CLP) , expanded if necessary
|
from Regulation (EC) No. 1272/2008 (CLP) , expanded if necessary
|
from Regulation (EC) No. 1272/2008 (CLP) , expanded if necessary
|
|||||||||
| H and P phrases | 226-304-312-315-319-335-412 | 226-304-312-332-315-319-335-412 | 226-304-312-315-332-319-335-412 | |||||||||
| 210-261-273-301 + 310-302 + 352-312-331 | 261-273-280-301 + 310-305 + 351 + 338-331 | 210-280-305 + 351 + 338-301 + 330 + 331 | ||||||||||
| MAK | Switzerland: 100 ml m −3 or 435 mg m −3 | |||||||||||
properties
Xylenes are colorless liquids that are hardly soluble in water (0.2 g / l). However , they show good solubility in ethers , alcohols , benzene and acetone . Aqueous solutions already show a recognizable taste in the concentration range from 0.53 to 1.8 ppm . Xylenes have typical aromatic smells; the odor threshold is between 0.2 and 174 mg / m 3 . The boiling points of the three isomers are close to one another, while their melting points differ significantly. The p -xylene has, such as. B. benzene or cyclohexane , due to its high symmetry a comparatively high melting point. The density is 0.86-0.88 g / cm 3 ; so the xylenes are all lighter than water. The flash point is around 30 ° C (depending on the isomer), the ignition temperature between 463 and 528 ° C. They burn with a very sooty flame.
Extraction and presentation
Raw material sources for the extraction of the xylenes are coal (from coal tar ) and petroleum (through cracking ), whereby isomer mixtures arise. Since the isomers have similar boiling points, separation by distillation is difficult. The separation of the o -isomer is carried out industrially by rectification , then p -Xylene can be separated by freezing out due to its relatively high melting point. On an industrial scale, the separation of the p- xylene with the aid of adsorption with a molecular sieve plays an important role.
use
Xylenes are used as solvents and are used in the manufacture of plastics and adhesives. Since the flash point of xylene is above 21 ° C, in practice it is one of the most important solvents for paints alongside butyl acetate . They are also added to fuels to increase the octane number .
p -Xylene is the raw material for terephthalic acid , which is used to manufacture saturated polyesters . o -Xylene is used to extract phthalic acid , mostly for the production of synthetic resins or synthetic fibers. By nitration gives the nitroxylenes caused by subsequent reduction to represent the xylidenes serve.
In addition to toluene , xylenes are among the technically most important aromatic hydrocarbons, the so-called BTEX aromatics.
Reactions
The methyl groups (-CH 3 ) can be oxidized to carboxy groups. Suitable oxidizing agents for converting both methyl groups are, for example, potassium permanganate or chromosulfuric acid . O -Xylene is converted into phthalic acid , m -Xylene into isophthalic acid and p -Xylene into terephthalic acid . o -Xylene can catalytically - z. B. Vanadium (V) oxide - are oxidized in the gas phase to phthalic anhydride . Oxygen (e.g. in the presence of cobalt stearate as a catalyst ) gives the corresponding monocarboxylic acids, the toluic acids (methylbenzoic acids). The benzene dicarbaldehydes can also be obtained by selective oxidation . Xylenes enter into the substitution reactions typical of aromatics .
hazards
Xylenes are flammable and are harmful to health when absorbed through the skin and the respiratory tract. For example, they can cause headaches, memory and orientation disorders, dizziness and shortness of breath. Xylenes are hazardous to water ( WGK 2 ). They form explosive mixtures between an air volume fraction of 1 to 8%. Xylene emissions are mainly due to motor vehicle traffic. In recent years there has been a decrease in xylene emissions.
The xylenes were included in the EU's ongoing action plan ( CoRAP ) in 2013 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of xylenes were concerns about consumer use , cumulative exposure , high (aggregated) tonnage, high risk characterization ratio (RCR) and widespread use as well as the dangers arising from a possible assignment to the group of CMR substances and the possible danger through sensitizing properties. The reassessment is to be carried out by Germany from 2020 .
literature
- Evaluation of toluene and xylene immissions. Erich Schmidt Verlag, Berlin (2000), ISBN 3-503-04071-4 .
Web links
- Hedinger: Safety data sheet xylenes . (PDF file; 197 kB)
Individual evidence
- ↑ a b c d e f Entry on xylene, mixture of isomers in the GESTIS substance database of the IFA , accessed on December 25, 2019(JavaScript required) .
- ↑ a b c d e f Entry on o-xylene in the GESTIS substance database of the IFA , accessed on December 25, 2019(JavaScript required) .
- ↑ a b c d e f Entry on m-xylene in the GESTIS substance database of the IFA , accessed on December 25, 2019(JavaScript required) .
- ↑ a b c d e f Entry on p-xylene in the GESTIS substance database of the IFA , accessed on December 25, 2019(JavaScript required) .
- ↑ Entry on o-xylene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on m-xylene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on p-xylene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 1330-20-7 or xylene (all isomers) ), accessed on September 14, 2019.
- ^ Bertram Philipp, Peter Stevens: Grundzüge der Industrielle Chemie , VCH Verlagsgesellschaft mbH, Weinheim 1987, ISBN 3-527-25991-0 , p. 181.
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Xylene , accessed on May 20, 2019.


