Furanoses
As furanoses refers lactols that a five-membered ring of four carbon - and an oxygen atom as well as at one of the oxygen bridge adjacent carbon atom a hydroxyl group contained. The ring is formed in certain monosaccharides by intramolecular hemiacetal formation between the carbonyl group and an OH group with the formation of a five-membered ring with one oxygen atom and four carbon atoms as ring members.
| Furan, tetrahydrofuran and furanose | |||
| Surname | Furan | Tetrahydrofuran | α- D -Altrofuranose |
| Structural formula | 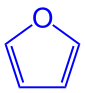 |
 |

|
| comment | Furan ring ( blue ) | Tetrahydrofuran ring ( blue ) | Tetrahydrofuran ring marked in blue |
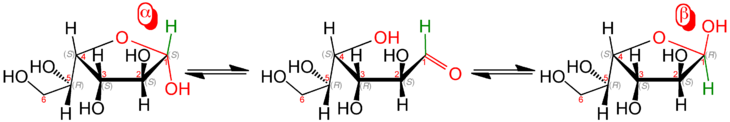
In the case of all monosaccharides with at least four carbon atoms, there is an equilibrium of open-chain and cyclic form in aqueous solution, the ring form being more stable. Rings with five atoms are called furanoses, rings with six atoms are called pyranoses . Higher molecular carbohydrates ( di- , oligo- and polysaccharides ) are also built up from ring-shaped monomers .
The name is derived from the heterocyclic molecule furan , which, however, also contains two double bonds in the ring system , so - in contrast to the furanoses - is an aromatic.
Nomenclature of furanoses
The atoms of the five-membered ring (tetrahydrofuran ring) are numbered in such a way that the anomeric carbon atom (original carbon atom of the carbonyl group of the aldose or ketose ) is always assigned the position 1. The position of the hydroxyl groups then results from the locant of the carbon atom to which this is bound in the acyclic form. Further conventions are described in the scientific literature.
| α-furanose and β-furanose of selected D aldoses |
| D - Allose (center) and the two forms of furanose |
| D - Altrose (middle) as well as its two forms of furanose |
| D - glucose (middle) and its two forms of furanose |
| D - mannose (center) and both forms of furanose |
| D - Gulose (middle) as well as its two forms of furanose |
| D - Idose (middle) and both forms of furanose |
| D - galactose (middle) as well as its two forms of furanose |
| D - Talose (middle) and both forms of furanose |
Individual evidence
- ^ Siegfried Hauptmann : Organic chemistry. 2nd revised edition. VEB Deutscher Verlag für Grundstofftindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 638.
- ↑ Furhop, JH; Endisch, C .: Molecular and Supramolecular Chemistry of Natural Products and Their Model Compounds . CRC Press, 2000, ISBN 0-8247-8201-1 .