Tropanols
The tropanols are derived from tropane , with a hydroxy substitution at the C-1, C-2 or C-3 atom. Two stereoisomers can be distinguished for the 2- and 3-tropanol . In the α-tropanols, the hydroxyl group is axial , in the β-tropanols equatorial to the ring system.
| Tropanols | |||||
| Surname | ( 1R ) -1-tropanol | 2α-tropanol | 2β-tropanol | 3α-tropanol | 3β-tropanol |
| other names |
|
|
|
|
|
| Structural formula |

|

|

|
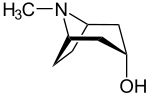
|

|
| CAS number | 36127-54-5 | 36127-15-8 | 120-29-6 | 28390-68-3 | |
| PubChem | 268139 | 54607705 | 8424 | ||
| Molecular formula | C 8 H 15 NO | ||||
| Molar mass | 141.21 g mol −1 | ||||
| Melting point | 47-48 ° C or 68-70 ° C | 46-48 ° C | −10 ° C | 63 ° C | 108-109 ° C |
| boiling point | 94-95 ° C | 229 ° C | 240-241 ° C | ||
properties
The (1 R ) -1-tropanol is in a tautomeric equilibrium with ( R ) - (methylamino) cycloheptanone .
Occurrence
Tropanols and tropane alkaloids derived from them occur in several plant families , especially in the nightshade family ( Solanaceae ). For example in henbane ( Hyoscyamus niger ), in deadly nightshade ( Atropa belladonna ) and in various thorn apple species ( Datura spec.). The (1 R ) -1-tropanol can be found in the Andean berries ( Physalis peruviana ). Cochlearin with tropine as an alcohol component is also found in the common spoonbill ( Cochlearia officinalis , Brassicaceae ). The other families are: Proteaceae , Convolvulaceae , Euphorbiaceae , Rhizophoraceae, and Erythroxylaceae . It could be proven that the development of the enzymes necessary for biosynthesis in nightshade and redwood plants took place independently of one another.
Individual evidence
- ↑ a b c d e f g Entry on 3α-tropanol. In: Römpp Online . Georg Thieme Verlag, accessed on January 29, 2013.
- ^ S. Sarel, E. Dykman: Hypoiodate-induced oxidative dimerization and functionalization of the tropane bicyclic system. In: Tetrahedron Lett. 17, 1976, pp. 3725-3728, doi: 10.1016 / S0040-4039 (00) 93093-4 .
- ^ A b D. E. Ayer, G. Büchi, PR Warnhoff, DM White: The structure of dioscorine. In: Journal of the American Chemical Society 80, 1958, pp. 6146-6146, doi: 10.1021 / ja01555a061 .
- ↑ a b W. AM Davies et al .: 703. An alkaloid of Dioscorea hispida, Dennstedt. Part VI. Some investigations on the synthesis of tropan-2-one. In: Journal of the Chemical Society . 1960, pp. 3504-3512, doi: 10.1039 / JR9600003504 .
- ↑ J. Jirschitzka, GW Schmidt et al .: Plant tropane alkaloid biosynthesis evolved independently in the Solanaceae and Erythroxylaceae. In: Proceedings of the National Academy of Sciences of the United States of America . Volume 109, Number 26, June 2012, pp. 10304-10309. doi: 10.1073 / pnas.1200473109 . PMID 22665766 . PMC 3387132 (free full text).

