( E , Z ) -Nona-2,6-dienal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
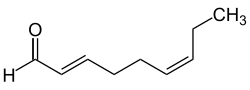
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | (E, Z) -Nona-2,6-dienal | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 14 O | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 138.21 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.86 g cm −3 (25 ° C) |
|||||||||||||||
| boiling point |
94–95 ° C (at 24 hPa) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.474 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
( E , Z ) -Nona-2,6-dienal (also called violet leaf aldehyde ) is a chemical compound from the group of aldehydes .
Occurrence
( E , Z ) -Nona-2,6-dienal is one of the main components for the aroma of sour cherries . It is also the main component of the extract obtained from the leaves of the scented violet (hence the common name violet leaf aldehyde ) and is also found in cooked trout , cucumber and some other plants. In cucumbers, it is the main component of the odor. The German scorpion fly releases the compound as a sex attractant.
Extraction and presentation
( E , Z ) -Nona-2,6-dienal can be obtained from α-linolenic acid .
properties
( E , Z ) -Nona-2,6-dienal is a colorless liquid that smells like cucumber.
use
( E , Z ) -Nona-2,6-dienal is used in the form of violet leaf extract in very low concentration (due to the strong smell) as a component of some perfumes.
Web links
- Zobrist, Fritz: On the constitution of violet leaf aldehyde. Dissertation, ETH Zurich, 1948 doi : 10.3929 / ethz-a-000090799 .
Individual evidence
- ↑ Entry on NONADIENAL in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b c d e f g h data sheet trans-2, cis-6-nonadienal, 95% from Sigma-Aldrich , accessed on May 6, 2012 ( PDF ).
- ↑ a b Entry on trans, cis-2,6-Nonadienal at TCI Europe, accessed on June 22, 2012.
- ↑ Entry on Nonadien-1-ale. In: Römpp Online . Georg Thieme Verlag, accessed on September 23, 2014.
- ↑ a b Horst Surburg, Johannes Panten: Common Fragrance and Flavor Materials: Preparation, Properties and Uses . John Wiley & Sons, 2006, ISBN 978-3-527-60789-1 , pp. 237 ( limited preview in Google Book search).
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance . Vieweg + Teubner, 2011, ISBN 978-3-8348-1245-2 , pp. 93, 102 ( limited preview in Google Book search).
- ^ Ralf G. Berger: Flavors and Fragrances: Chemistry, Bioprocessing and Sustainability . Springer, 2006, ISBN 978-3-540-49338-9 , pp. 172 ( limited preview in Google Book search).
- ↑ Journal of Chemical Ecology, Volume 33, Number 6 (2007), 1249–1256, doi : 10.1007 / s10886-007-9304-3 A Male Sex Pheromone in a Scorpionfly, Dagmar Kock, Joachim Ruther and Klaus Peter Sauer
- ↑ RH Buescher, Production and Stability of (E, Z) -2,6-Nonadienal, the Major Flavor Volatile of Cucumbers, 2006, doi : 10.1111 / j.1365-2621.2001.tb11346.x , Journal of Food Science, Volume 66 , Issue 2, pp. 357-361, March 2001.