Rhodopsin
| Rhodopsin | ||
|---|---|---|

|
||
| Three-dimensional structural model of the rhodopsin backbone . The 11-cis retinal, which is important for the signal cascade, can be seen in the middle (light gray) . According to PDB 1L9H . | ||
| Properties of human protein | ||
| Mass / length primary structure | 348 amino acids | |
| Secondary to quaternary structure | multipass membrane protein | |
| Identifier | ||
| Gene name | RHO | |
| External IDs | ||
| Occurrence | ||
| Parent taxon | Bilateral animals (opsine) | |
Rhodopsin (also called visual purple because of its color, especially in older textbooks and research papers ) is a light-sensitive receptor molecule . Rhodopsin is one of the visual pigments in the retina (retina) of the eyes of vertebrates (vertebrates), in the compound eyes of insects and in the photoreceptors of other invertebrates (invertebrates). In addition, rhodopsins (see bacteriorhodopsin and channelrhodopsin ) also occur in bacteria , archaebacteria and unicellular algae.
In humans and in most vertebrate eyes, the rhodopsin in the rods of the retina is responsible for light-dark vision at low brightness ( scotopic vision ). In contrast, color vision or day vision with the help of the cones is based on three, and in a number of animal species also four, different variants of iodopsin , a related visual pigment.
Details
Rhodopsin structure
Rhodopsin consists of a protein component, the rod opsin ( scotopsin ) and the covalently bound chromophore 11- cis - retinal . The 11- cis retinal (aldehyde of retinol ) is bound as an imine (Schiff's base) to the ε-amino group of the lysine unit K256 of the protein. Opsins are transmembrane proteins . In the rod cells of the retina, however, rhodopsin is not stored in the cell membrane, but is located in the membranes of disc-shaped organelles (discs) inside the cell.
Vertebrate rhodopsins are members of the large family of G protein-coupled receptors (GPCRs). Bovine rhodopsin was the first G-protein-coupled receptor, of which a crystal structure obtained by X-ray structure analysis was available (the seven helical transmembrane domains are characteristic, among other things - see figure). It therefore served as a template for models of other GPCRs, even if the agreement in the primary structure is sometimes very poor. The crystal structures of numerous other GPCRs are now available.
Processes in the activation of rhodopsin by light
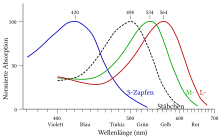
The absorption maximum of rhodopsin in the visible light wavelength range is λ = 500 nm. Absorption of a single photon in the appropriate energy range leads in the 11- cis -retinal to an isomerization after all-trans -retinal. This changes the spatial structure of the retinal, and internal interactions in the molecule lead to a series of conformational changes in the protein portion of the pigment, which transform the rhodopsin into a metastable active state called Meta II. In research, one speaks of "bleaching" ("bleaching"), as the pigment loses its reddish color in the course of activation. The altered functional properties of activated rhodopsin are the basis for a number of rapidly occurring changes in the cell.
Vertebrate rhodopsins are G-protein coupled receptors. Light-activated rhodopsin in the rod cells of the retina is capable of activating the G-protein transducin and thereby triggers visual signal transduction , a multi-step reaction cascade , the end point of which is a change in the transmembrane voltage, which is expressed in the "language" of nerve cells, for transmission in the nervous system suitable electrical signal, and in the course of which the original excitation is amplified many times over.
Microbial rhodopsins, on the other hand, are often light-activated proton pumps , ion pumps or ion channels located in the cell membrane : their activation results directly in an electrical signal without any intermediate steps.
Processes of rhodopsin deactivation and regeneration
The metastable Meta II state also spontaneously turns back into an inactive state. Usually - and especially in the context of visual transduction in the rod cells of the retina - activated rhodopsin is deactivated by a faster enzymatic process - phosphorylation by the enzyme rhodopsin kinase and binding of the protein arrestin. In the course of the deactivation, the all-trans retinal is released. To regenerate the light-sensitive rhodopsin, 11- cis -retinal has to be bound again. This also involves complex, enzymatically controlled processes: The “recycling” from all-trans -retinal to 11- cis -retinal takes place outside the cell in the adjacent retinal pigment epithelium .
Medical reference
Mutations in the opsin gene can lead to retinitis pigmentosa and hereditary night blindness .
A lack of vitamin A as a source of the retinal leads to night blindness , dryness of the eye (xerophthalmia) and corneal inflammation (keratitis) of the eye. Vitamin A deficiency can lead to blindness in children. This is particularly common in developing countries because of the rice-based diet . The daily requirement of an adult for vitamin A is set at 800 µg in accordance with European Directive 90/496 / EEC (EU nutrition labeling directive ).
Related topics
A similar molecule, bacteriorhodopsin , is found in halobacteria . It also contains retinal and is also made up of seven transmembrane domains. However, it is not linked to a G protein. It is a light-driven proton pump .
In higher green plants, on the other hand, phytochrome functions as a light receptor, which, like rhodopsin, can occur in different states and thus gives the plant information about the currently existing light conditions.
See also
Web links
Individual evidence
- ↑ Search result UniProt Opsins by Taxonomy .
- ^ A b c Werner A. Müller, Stephan Frings, Frank Möhrlen: Animal and human physiology: An introduction . Springer Spectrum, Berlin 2019, ISBN 978-3-662-58461-3 , Der Sehsinn, p. 605 ( [1] ).
- ↑ Christina Beck: Protozoa shed light on neurobiology. www.mpg.de, November 20, 2014, accessed on October 2, 2019 .
- ^ A b H. Gobind Khorana: Rhodopsin, Photoreceptor of the Rod Cell . In: Journal of Biological Chemistry . 267, No. 1, 1992, pp. 1-4.
- ↑ SB: Ghakasan et al .: G protein-coupled receptors: the evolution of structural insight . In: AIMS Biophysics . 4, No. 3, 2017, pp. 491-527. PMC 6018013 (free full text).
- ^ Bowmaker & Mollon: Human rods and cones . 1983, table of values at 500 nm ( Color and Vision Research Labs )
- ↑ D. Baylor: How photons start vision . In: Proceedings of the National Academy of Sciences USA . 93, No. 2, 1996, p. 560. PMC 40091 (free full text).
- ^ PD Kieser et al .: Chemistry of the Retinoid (Visual) Cycle . In: Chemical Reviews . 114, No. 1, 2014, pp. 194-232. PMC 3858459 (free full text).
- ↑ Directive 2008/285 / EC (PDF) of the Commission of October 28, 2008 amending Council Directive 90/496 / EEC on the nutritional labeling of foods with regard to the recommended daily allowances, the conversion factors for the energy value and the definitions.