Laminaribiosis
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
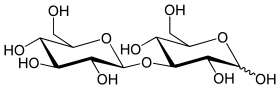
|
||||||||||
| General | ||||||||||
| Surname | Laminaribiosis | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 12 H 22 O 11 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 342.30 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
205 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Laminaribiose is a chemical compound from the group of disaccharides ( double sugar ). The substance consists of two molecules of glucose that are linked by a β (1 → 3) - glycosidic bond . It arises as a partial breakdown product of laminarin from brown algae . Laminaribiosis is also found in some land plants as a component of glycosides , for example in the timeless Colchicum speciosum with the aglycon luteolin . The disaccharide was also found in honey and beer ; Barley malt contains the enzyme laminarinase ( endo -1,3 (4) -β-glucanase), which can break down β (1 → 3) - and β (1 → 4) -linked glucans in yeast .
Individual evidence
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-318.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Jeffrey B. Harborne, Herbert Baxter, Gerard P. Moss: Phytochemical dictionary: a handbook of bioactive compounds from plants. 2nd edition, CRC Press, 1999, ISBN 978-0-7484-0620-3 , p. 12.
- ↑ H.-D. Belitz , W. Grosch, P. Schieberle: Textbook of food chemistry. 6th edition, 2007, Springer, ISBN 978-3-540-73201-3 , p. 915.
- ^ Gerhard G. Habermehl, Peter E. Hammann, Hans C. Krebs, W. Ternes: Naturstoffchemie: An introduction. 3rd edition, Springer, 2008, ISBN 978-3-540-73732-2 , p. 387.
- ↑ H.-D. Belitz, W. Grosch, P. Schieberle: Textbook of food chemistry. 6th edition, 2007, Springer, ISBN 978-3-540-73201-3 , p. 341.