Ferulic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
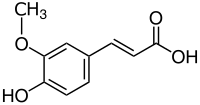
|
||||||||||||||||||||||
| Structural formula of trans -ferulic acid | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Ferulic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 10 H 10 O 4 | |||||||||||||||||||||
| Brief description |
yellowish odorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 194.19 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.14 g cm −3 |
|||||||||||||||||||||
| Melting point |
169-173 ° C |
|||||||||||||||||||||
| solubility |
soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ferulic acid (more precisely trans -Ferulasäure ) is an organic compound , the (or its ester ) in various plants (eg. B. Stinkasant , dill , rice , grasses) occurs. See also the plant genus Ferula . It plays a role in the synthesis of lignin in the cell walls of plants.
Chemical properties
Ferulic acid belongs to the group of phenolic acids . The curcumin of turmeric is derived from its structure . Of course, it occurs almost exclusively in the trans form. Only beans contain a higher proportion of cis -ferulic acid, with some researchers assuming that the cis -form only arises when the plant samples are stored.
use
Ferulic acid is used as a raw material for the production of vanillin and antimicrobial substances for soaps, fragrances and cosmetics.
Importance in beer preparation
Ferulic acid has a decisive influence on the later sensory characteristics of the beer , especially in the preparation of wheat beer.
Occurrence of ferulic acid: Ferulic acid is bound to the insoluble pentosans in malt , there to arabinose side chains. Barley malt has a higher proportion of bound ferulic acid than wheat malt; analogously, higher values are found in barley malt seasonings.
Influence during mashing : During mashing, there is the greatest influence on the release of ferulic acid (during malting only through hydrolytic pentosan degradation). Favorable mashing temperatures of 37–47 ° C cause a significant increase in ferulic acid, 44 ° C is an optimal temperature. Through the breakdown of the arabinoxylan by the endo- and exo-xylanases (optimum 45 ° C) and arabinosidases (40–45 °) C) the ferulic acid is released. A reduction in the mash pH below 5.7 slows down the degradation.
Fermentation : Top-fermenting yeast has the property of forming 4-vinylguajacol from ferulic acid as a precursor, which is a typical wheat beer aroma and is reminiscent of cloves. During fermentation, the ferulic acid is decarboxylated to form 4-vinylguaiacol, in wheat beer yeast in amounts of approx. 0.5–3.0 ppm. The formation is less due to the usual temperatures of 15-25 ° C during fermentation than to the vessels and yeast systems used. Multiple yeast management by harvesting in the upright cylinder-conical fermentation tank has a negative impact on the formation of 4-vinylguaiacol due to increased static pressure, which also has a general effect on the formation of esters and stress on the yeast cells. The desirable range of 4-vinylguaiacol formed is 1.2-1.7 ppm.
Individual evidence
- ↑ Entry on FERULIC ACID in the CosIng database of the EU Commission, accessed on May 13, 2020.
- ↑ a b c d e Entry on ferulic acid in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Carl L. Yaws; Thermophysical Properties of Chemicals and Hydrocarbons; ISBN 978-0-8155-1596-8 .
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 385, ISBN 978-3-906390-29-1 .
- ↑ Tokusoglu Ozlem: Fruit and Cereal Bioactives: Sources, Chemistry, and Applications . CRC Press, 2011, ISBN 978-1-4398-0665-4 ( page 63 in the Google book search).
- ↑ Cesar G. Fraga: Plant phenolics and human health . Wiley, 2009, ISBN 978-0-470-28721-7 ( page 67 in the Google book search).
