Curcumin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Keto form (top) and enol form (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Curcumin | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 21 H 20 O 6 | |||||||||||||||||||||
| Brief description |
orange-yellow solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 368.39 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
183 ° C |
|||||||||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Curcumin is an intense orange-yellow (but not lightfast ) natural dye .
Occurrence and extraction
Curcumin is found in the rhizome of the turmeric plant ( Curcuma longa , also called turmeric ). The name of the dye is derived from this occurrence. However, it can also be produced synthetically.
chemistry
properties
Curcumin has several tautomeric forms and configurational isomers : a keto form, an all-trans and a cis, trans isomer.
Curcumin dissolves in acid with a light yellow color and in alkaline brown-red. The transition point is between pH 8 and 9. Curcumin, as a polyene, is light-sensitive. When excited, it exhibits broadband self-fluorescence.
Curcumin can be used as a reagent for the detection of boron in the form of borates , since the red dye rosocyanine is formed in acidic solution or the dye rubrocurcumin is formed in the presence of oxalic acid .
synthesis
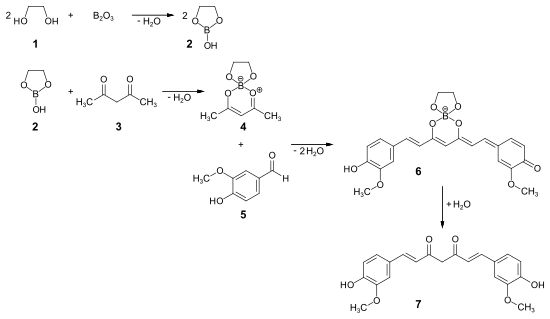
Synthesis pathways for curcumin are summarized in monographs from 2003 and 2007, whereby multilevel possibilities are also considered. However, only one-step syntheses from vanillin and acetylacetone by means of aldol condensation are of interest , whereby the stability of the boric acid complexes is used. The incorporation of the more reactive methylene group in acetylacetone in a 1,3,2-dioxaborolanol system in the enol form of 1,3- diketone blocks this position in favor of the two methyl groups. The water formed in the aldol condensation leads to hydrolysis of the pseudo-aromatic complex, which in turn reduces the regioselectivity of the reaction. Several ways of removing the water formed are used. The first one-step synthesis was achieved in 1950 by melting the components together. In 1985 the use of boron oxide was described.
In the patent literature as early as 1962, either a large excess of boric acid ester at high dilution was used, or again in the melt or in dimethyl sulfoxide , resulting in highly viscous mixtures. Only the complexation of the boric acid with a glycol to a soluble 1,3,2-dioxaborolanol, reaction with acetylacetone to a spirocyclic boric acid ester, which with vanillin with removal of the water of reaction during the aldol condensation by azeotropic rectification with a suitable entrainer results in the deep red boric acid complex of curcumin as precipitation. This reaction procedure can reduce the hydrolysis of the boric acid complexes and thus the side reactions. Curcumin is released through targeted hydrolysis of the precipitation (see reaction scheme).
Bioavailability
Since curcumin is sparingly soluble in water, it is only absorbed to a very small extent in the gastrointestinal tract. Heating or dissolving in oil increases the bioavailability of curcumin found in foods.
Using adsorption mediators, several approaches for increased bioavailability are being investigated. A curcumin- phospholipid complex has a 29-fold higher bioavailability than conventional curcumin. Black pepper extract ( piperine ) causes a 20-fold bioavailability of curcumin and is used in most nutritional supplements with curcumin. Various approaches to improving the bioavailability of curcumin formulations are summarized in a recent online review article, with preparations containing curcumin dispersed in hydrophobic carriers producing the best results. Also cyclodextrin is suitable as a carrier.
use
Curcumin is widely used as a food additive E 100 for coloring food, e.g. B. margarine , pasta , potato flakes , rice - ready meals, jam , jam and mustard . Curcumin is also increasingly used in dietary supplements .
In addition, curcumin is also a flavor carrier of turmeric, which is used as a spice and flavoring substance . The powder ground from the rhizome is a traditional and essential component of curry powder .
Its use as a textile dye was discontinued because of its instability in alkaline media.
Curcumin accumulates on β-amyloid , the smallest traces of which in the peripheral areas of the retina - years before the development of the associated Alzheimer's disease - can be detected by an eye scan.
Medical aspects
In vitro studies
Since curcumin, one of the strongest pan-assay interference compounds (PAINS), can cause false-positive results in chemical tests (e.g. high-throughput screenings ), a large part of the published results from in-vitro studies must be questioned. Due to the low oral bioavailability of curcumin, the results of in vitro studies cannot be directly applied to humans. Numerous approaches are therefore aimed at increasing the bioavailability of curcumin formulations (see above). Turmeric powder contains many different components, so the effects shown in in vitro studies could also be caused by other components.
Clinical studies
While preclinical studies are of relatively little informative value, clinical studies describe the actual effects of curcumin on humans. Due to the low bioavailability of curcumin, the success of the clinical studies depends heavily on the curcumin formulation used .
Osteoarthritis and pain
A review study showed that curcumin can both reduce pain and improve mobility in osteoarthritis patients : The studies considered showed a highly significant reduction in the Pain Visual Analogue Score (PVAS) by curcumin compared with a placebo (P <.00001) and a Reduction in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (P = .009). In addition, no significant difference in PVAS was found between curcumin and pain medication ( ibuprofen , diclofenac , glucosamine ). This suggests that curcumin has similar pain relieving effects as these pain relievers. A curcumin-phospholipid was compared to chondroitin and the Karnofsky Index improved the patients.
Web links
- Monya Baker: Deceptive curcumin offers cautionary tale for chemists. In: Nature . January 11, 2017. doi: 10.1038 / 541144a
Individual evidence
- ↑ Yana Manolova, Vera Deneva, Liudmil Antonov et al: The effect of the water on the curcumin tautomerism: A quantitative approach . In: Spectrochimica Acta . tape 132 A, no. 1 , 2014, p. 815-820 , doi : 10.1016 / j.saa.2014.05.096 .
- ↑ Entry on E 100: Curcumin in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on CURCUMIN in the CosIng database of the EU Commission, accessed on August 6, 2020.
- ↑ a b Entry on curcumin I. In: Römpp Online . Georg Thieme Verlag, accessed on February 14, 2019.
- ↑ a b Entry on curcumin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c Curcumin data sheet from Sigma-Aldrich , accessed on March 23, 2011 ( PDF ).
- ^ VA Parthasarathy: Chemistry of Spices. CABI, 2008, ISBN 978-1-84593-420-0 , p. 104 ( limited preview in Google book search).
- ↑ G. Ciamician, P. Silber, On the knowledge of curcumin. In: Ber. d. German Chem. Ges. Volume 30, 1897, p. 192.
- ↑ Curcumin and synthetic derivatives as environment-sensitive fluorescence probes macau.uni-kiel.de
- ↑ Silvia Schmautz: Curcumin: Novel therapeutic applications of an old traditional drug. With focus on Alzheimer's Disease. University of Vienna, Department of Medicinal / Pharmaceutical Chemistry, Faculty of Life Sciences, 2007 (English, 80 pp., Full text [PDF] Diploma thesis for obtaining the academic degree Magistra Pharmaciae).
- ↑ Dirk Rohde: Presentation and property investigations on 1,3,2-dioxaborines with variable coligands on the boron atom. sundoc.bibliothek.uni-halle.de
- ^ T. Pavolini, F. Gambarin, AM Grinzato: Curcumina e curcuminoidi. In: Ann.Chimica. (Rome). Volume 40, 1950, p. 280; Chem. Abstr. Volume 52, 1951, p. 816. (books.google.de)
- ↑ Uffe Pedersen, Preben B. Rasmussen, Sven-Olov Lawesson: Synthesis of Naturally Occurring Curcuminoids and Related Compounds. In: EurJOC. 1985, p. 1557. doi: 10.1002 / jlac.198519850805
- ↑ Jan van Alphen, Hendrik JJ Pabon (Unilever): DE 1 204 765. Process for the production of dyes by reacting 2,4 -pentanediones with aldehydes.
- ↑ Erich Graf (Ludwig Heumann & Co): DE 1 280 849. Process for the production of curcumin from vanillin and acetylacetone.
- ↑ Erich Graf (Ludwig Heumann & Co): DE 1 282 642. Process for the production of curcumin from vanillin and acetylacetone.
- ^ Clark G. Spike (Standard Oil Co.): US 2,961,459. Glycol pyroborates, 1960.
- ↑ W. Liebenow, I. Grafe (Heumann & Co GmbH): DE 2 501 220. One-step process for the production of substituted 1,7-diphenyl-5-hydroxy-hepta- (1,4,6) -triene- (3) -onen, 1975.
- ↑ Tonnesen include: Studies of curcumin and curcuminoids XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. In: Int J Pharm . Volume 244, 2002, pp. 127-135. PMID 12204572 .
- ↑ Pan et al: Biotransformation of curcumin through reduction and glucuronidation in mice. In: Drug Metab Dispos . Volume 27, 1999, pp. 486-494. PMID 10101144 .
- ↑ TH Marczylo, RD Verschoyle, DN Cooke, P. Morazzoni, WP Steward, AJ Gescher: Comparison of systemic availability of curcumin with curcumin did of Formulated with phosphatidylcholine. In: Cancer Chemotherapy and Pharmacology . Volume 60, No. 22, 2007, pp. 171-177.
- ↑ P. Anand, AB Kunnumakkara, RA Newman, BB Aggarwal: Bioavailability of curcumin: problems and promises. In: Molecular Pharmaceutics. Volume 4, No. 6, 2007, pp. 807-818.
- ↑ Cuomo et al .: Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. In: J Nat Prod. Volume 74, No. 4, Apr 25, 2011, pp. 664-669. PMID 21413691 .
- ↑ NK Gupta, VK Dixit: Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. In: J Pharm Sci. Volume 100, No. 5, May 2011, pp. 1987-1995. PMID 21374628 .
- ↑ G. Shoba, D. Joy et al .: Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. In: Planta Med. Volume 64, No. 4, May 1998, pp. 353-356.
- ↑ Brad J. Douglass, Dallas L. Clouatre: Beyond Yellow Curry: Assessing Commercial Curcumin Absorption Technologies. In: Journal of the American College of Nutrition . tape 34 , no. 4 , 2015, p. 347-358 , doi : 10.1080 / 07315724.2014.950392 , PMID 25856323 (English).
- ^ M. Purpura, RP Lowery, JM Wilson, H. Mannan, G. Münch, V. Razmovski-Naumovski: Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. In: Eur J Nutr . online, 2017, doi : 10.1007 / s00394-016-1376-9 , PMID 28204880 (English).
- ↑ Y. Koronyo et al ., Retinal amyloid pathology and proof-of-concept trial imaging disease in Alzheimer's, JCI Insight 2 (2017), doi: 10.1172 / jci.insight.93621
- ↑ Photonics: Alzheimer's diagnosis by eye scan , Photonics , September 28, 2017, accessed on August 7, 2020
- ↑ Kathryn M. Nelson, Jayme L. Dahlin, Jonathan Bisson, James Graham, Guido F. Pauli, Michael A. Walters: The Essential Medicinal Chemistry of Curcumin . In: Journal of Medicinal Chemistry . January 11, 2017, doi : 10.1021 / acs.jmedchem.6b00975 .
- ↑ Monya Baker: Deceptive curcumin offers cautionary tale for chemists . In: Nature . tape 541 , no. 7636 , January 9, 2017, p. 144-145 , doi : 10.1038 / 541144a .
- ↑ James W. Daily, Mini Yang, Sunmin Park: Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials . In: Journal of Medicinal Food . tape 19 , no. 8 , August 1, 2016, p. 717-729 , doi : 10.1089 / jmf.2016.3705 , PMID 27533649 .