Chondroitin
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Chondroitin sulfate C |
|||||||||
| General | |||||||||
| Surname | Chondroitin | ||||||||
| other names |
Poly [( N -acetyl- D -galactosamine-4- O- hydrogen sulfate) - ( L -iduronic acid)] ( IUPAC ) |
||||||||
| CAS number |
|
||||||||
| Monomers / partial structures | N-acetylgalactosamine and glucuronic acid | ||||||||
| Type of polymer | |||||||||
| ATC code | |||||||||
| Drug information | |||||||||
| Drug class |
Anti-arthrotic |
||||||||
| properties | |||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Chondroitin sulfate (also chondritin sulfate ) is a biological macromolecule . The substance formed by the chondroblasts is an important part of the cartilage tissue and contributes to its resistance to compression.
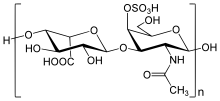
Chemically, it is a mixture of sulfated glycosaminoglycans (GAG), also called mucopolysaccharides . In the polymer chains , the sugar derivatives N- acetylgalactosamine (GalNAc) and glucuronic acid alternate.
Chemical structure
Chondroitin sulfate chains are unbranched polysaccharides of variable length. They always consist of two alternating simple sugars: D- glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc). Here the glucuronic acid is linked with GalNAc β-1 → 3 and GalNAc to form glucuronic acid β-1 → 4-glycosidically. A chondroitin chain can consist of over 100 sugar units, which vary in strength and can be sulfated at positions C6 and / or C4 of the GalNAc. If the glucuronic acid epimerizes at C5 to iduronic acid, we speak of dermatan sulfates. Understanding the striking diversity of chondroitin sulfate and the related glycosaminoglycan is one of the goals of glycobiology .
Sulfation
Each simple sugar can be present with either no, one or two sulphate groups. Most often the hydroxyl groups on carbon atoms 4 and 6 of N- acetyl-galactosamine are provided with a sulfate group. The sulfation is catalyzed by various sulfotransferases . The degree of sulfation averages 0.8 based on the disaccharide.
A distinction is made between the following types:
| Chondroitin type | Systematic name | Disaccharide unit | description |
| Chondroitin Sulphate A | Chondroitin 4 sulfate | GlcA-GalNAc4S | Sulphate groups mainly on carbon atom 4 of the N -acetylgalactosamine (GalNAc) |
| Chondroitin sulfate B | Dermatan sulfate | IdoA2S-GalNAc4S | Sulphate groups mainly on carbon atom 2 of iduronic acid and on carbon atom 4 of GalNAc |
| Chondroitin sulfate C | Chondroitin 6 sulfate | GlcA-GalNAc6S | Sulphate groups mainly on carbon atom 6 of GalNAc |
| Chondroitin sulfate D. | Chondroitin-2,6-sulfate, dermatan sulfalt | GlcA2S-GalNAc6S | Sulphate groups mainly on carbon atom 2 of glucuronic acid and on carbon atom 6 of GalNAc (chondroitin-2,6-sulfate) |
| Chondroitin sulfate E. | Chondroitin-4,6-sulfate | GlcA-GalNAc4,6diS | Sulfate groups mainly on carbon atoms 4 and 6 of GalNAc |
The typing by the letters A to E comes from the time when chondroitin sulfate was already isolated, but the structure was not yet known. The classification of dermatan sulphate under the chondroitin sulphates as chondroitin sulphate B is now regarded as a misnomer, since this compound is not derived from glucuronic acid but from iduronic acid. The most common chondroitin sulfates are types A, B and C.
The formerly common name chondroitin without “sulfate”, which was used to describe a chain with no or very few sulfate groups, is no longer common.
Although the name suggests that chondroitin sulfate salt with a sulphate - anion is, the sulphate covalently linked to the sugar. Since the molecule has several negative charges at physiological pH , it is present as an anion in the salts of chondroitin sulfate. The commercial product of chondroitin sulfate is usually its sodium salt. Barnhill et al. have suggested that all chondroitin sulfate products should be labeled as "sodium chondroitin" regardless of their sulfate linkages.
A synthetically re-sulfated variant is chondroitin polysulfate (“oversulfated chondroitin sulfate”, English “oversulfated chondroitin sulfate”, OSCS ). The additional sulfation increases the anti-inflammatory ( anti-inflammatory ) effect of the substance. Chondroitin polysulphate also has anticoagulant ( anticoagulant ) and fibrinolytic activity.
Protein binding
Biologically, chondroitin sulfate is usually bound to proteins, as part of a proteoglycan . The chondroitin sulfate chains are bound to the hydroxyl groups of the serine residues of certain proteins. How exactly the proteins are selected for binding with glycosaminoglycans is not yet understood. Glycine residues are often followed by glycine residues, with acidic amino acid residues in the vicinity.
The link in the GAG chain is always the same sugar chain made up of three sugar units:
| Ser–O–Xyl−Gal−Gal−GlcA-… |
Each sugar is bound by a specific enzyme, which allows complex control over GAG synthesis. Xylose is bound to proteins in the endoplasmic reticulum , while the rest of the sugars are transferred in the Golgi apparatus .
function
The function of chondroitin is highly dependent on the properties of the total proteoglycan to which it belongs. Chondroitin proteoglycans have both structural and regulatory roles.
Structural function
Chondroitin proteoglycans, together with collagen, are essential components of the extracellular matrix . They ensure the structural integrity of the tissue. Typical representatives are Aggrecan , Versican , Brevican and Neurocan .
As part of aggrecan, chondroitin sulfate forms a large part of the cartilage mass. The tightly packed, highly charged sulfate groups lead to electrostatic repulsion of the individual chains (see Coulomb's law ), which causes a large part of the resistance of the cartilage to compression. Loss of chondroitin sulfate from the cartilage is one of the most common causes of osteoarthritis .
Regulatory function
Also due to the negative charges, chondroitin sulfate easily interacts with other proteins of the extracellular matrix. These interactions are important for the regulation of a large number of cellular processes. In comparison to heparan sulfate , another proteoglycan of the extracellular matrix, little is known about the role of chondroitin sulfate proteoglycans. It is known that chondroitin sulfate regulates the growth and development of the nervous system as well as its response to injury.
Extraction
Chondroitin sulfate is largely obtained from the cartilage tissue of cattle , pigs and sharks . Other types of fish and birds can also provide cartilage.
use
Cartilage regeneration, cartilage protection
Chondroitin sulfate is used medicinally and dietetically in the treatment of degenerative joint diseases ( osteoarthritis ) e.g. B. the hip, the knee or the fingers. Chondroitin sulfate is also taken as a dietary supplement to keep the cartilage healthy and to prevent arthrotic signs of wear and tear (chondroprotection). It is often combined with glucosamine . The application is based on the idea that the supplied chondroitin sulfate is "built into" the cartilage. In vitro (ie outside a living organism) chondroitin sulfate has an anti-inflammatory effect.
Orally, chondroitin sulfate is usually used in doses of 800–1200 mg per day. In 2007, the Federal Institute for Risk Assessment could not derive a dose from which pharmacological efficacy occurred due to insufficient data. Since chondroitin is not a uniform substance and it occurs naturally in a wide range of different forms, the composition of finished products varies accordingly. There are no binding standards for composition in the food sector. In the US, it was discovered in 2000 that a high proportion of chondroitin-containing food supplements were incorrectly or misleadingly labeled with regard to chondroitin content.
There are no valid data on the oral bioavailability of chondroitin sulfate and its metabolism.
- effectiveness
- In meta-studies, the significant improvement of certain target parameters (e.g. Lequesne index , WOMAC index , VAS ) was found during osteoarthritis treatment with chondroitin sulfate.
- In 2006, the US National Institutes of Health (NIH) initiated a multicenter, placebo-controlled , six-month blind study on the effectiveness of chondroitin and glucosamine in osteoarthritis of the knee. It found no statistically significant effect on osteoarthritis symptoms in patients with milder pain. Joint swelling was reduced. Treatment effects were found in a subgroup of patients with moderate to severe pain, but the informative value was not conclusive due to the small number of patients in this subgroup.
- The result of more recent meta studies (2007 & 2010) was that chondroitin, glucosamine, and their combination have no clinically relevant effects on the perceived joint pain or on joint wear.
- A double-blind, randomized, placebo-controlled study from 2013, carried out by the University of Sydney with 605 test subjects (age 45–75 years, knee joint osteoarthritis ) was able to observe a statistically significant weakening of the receding joint space . For 2 years, 2 × 750 mg glucosamine sulfate and 2 × 400 mg chondroitin sulfate were taken daily. A reduction in joint pain was seen in all groups [1) glucosamine sulfate 2 × 750 mg, 2) chondroitin sulfate 2 × 400 mg, 3) glucosamine sulfate 2 × 750 mg + chondroitin sulfate 2 × 400 mg, 4) placebo] - without significant differences between the groups - to be watched.
- A six-month double-blind study in knee osteoarthritis patients compared the effects of 400 mg chondroitin sulfate plus 500 mg glucosamine sulfate three times a day with 200 mg celecoxib , a COX-2 inhibitor, once a day . Both treatments were comparably efficient in terms of pain reduction, mobility and swelling after six months. Celecoxib, however, had a stronger effect than chondroitin sulfate and glucosamine sulfate in the months before.
- safety
In the usual oral doses of up to 1200 mg per day, chondroitin sulfate is well tolerated. No serious side effects from chondroitin sulfate were found in clinical studies in this dose range.
The intramuscular and intra-articular use of chondroitin sulfates has been replaced by better tolerated hyaluronates . A decades-applied intraarticularly as osteoarthritis remedy chondroitin preparation was 1992 because of severe anaphylactic and thrombo embolic taken side effects from the market.
Ophthalmology and urology
Aqueous chondroitin sulfate solutions are used in ophthalmology , in combination with hyaluronate, during operations on the eye in order to wet and protect the corneal endothelium . As a bladder rinsing solution, chondroitin sulfate is used to restore the bladder mucosa, for example if it is damaged by chronic inflammation ( interstitial cystitis ).
Local anti-inflammatory use
One area of application for chondroitin polysulphate (“oversulphated chondroitin sulphate”) is the topical treatment of blunt injuries and superficial phlebitis . In animal tests and skin models has been shown that chondroitin polysulfate able to penetrate through the skin into the underlying tissue. There it should develop its anti-inflammatory ( anti-inflammatory ) effect. Chondroitin polysulfate is applied as an ointment , cream, or gel.
Heparin scandal
In 2008, the incident known as the “ heparin scandal ” occurred after Chinese drug manufacturers “diluted” the drug heparin with cheaper oversulfated chondroitin (chondroitin polysulfate). As a result, numerous serious side effects, some of which were fatal, occurred.
Commercial preparations
- Chondroitin sulfate
- Monopreparations: Condrosulf (A, CH), Gepan instill (D), Structum (CH)
- Combination preparations with additional nutrients: Glusatin (D, A), Arthrovitan (D), Arthrobel (D), CondroTect (D), Duovital (D), Orthomol Arthro (D), Orthoexpert (D), various food supplements
- Combination preparations with hyaluronic acid : ProVisc (D), VisCoat (D), various food supplements
- Chondroitin polysulfate
Hirudoid (D), combination with salicylic acid : Mobilat DuoAktiv (D)
See also
Web links
- "NIH News: Efficacy of Glucosamine and Chondroitin Sulfate May Depend on Level of Osteoarthritis Pain," February 22, 2006.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Baeurle SA, Kiselev MG, Makarova ES and Nogovitsin EA: Effect of the counterion behavior on the frictional-compressive properties of chondroitin sulfate solutions . In: polymer . 50, 2009, pp. 1805-1813. doi : 10.1016 / j.polymer.2009.01.066 .
- ^ Fotini N. Lamari and Nikos K. Karamanos: Structure of Chondroitin sulfate . P. 34; In: Nicola Volpi (Ed.): Chondroitin Sulfate: Structure, Role and Pharmacological Activity (Advances in Pharmacology) . Academic Press Inc 2006; ISBN 978-0-12-032955-7
- ^ PA Levene and FB La Forge: On Chondroitin Sulphuric Acid . (PDF) In: J. Biol. Chem. . 15, 1913, pp. 69-79.
- ↑ Davidson EA, Meyer K: Chondroitin, a new mucopolysaccharide . (PDF) In: J Biol Chem . 211, No. 2, 1954, pp. 605-11. PMID 13221568 .
- ↑ Barnhill JG, Fye CL, Williams DW, Reda DJ, Harris CL, Clegg DO: Chondroitin product selection for the glucosamine / chondroitin arthritis intervention trial . In: J Am Pharm Assoc . 46, No. 1, 2006, pp. 14-24. PMID 16529337 .
- ↑ Silbert JE, Sugumaran G: Biosynthesis of chondroitin / dermatan sulfate . In: IUBMB Life . 54, No. 4, 2002, pp. 177-186. PMID 12512856 .
- ↑ Federal Institute for Risk Assessment : Use of Chondroitin Sulphate in Food Supplements , (PDF; 132 kB) Opinion No. 031/2007 of June 15, 2007.
- ↑ Adebowale AO Cox DS, Liang Z, Eddington ND: Analysis of glucosamine and chondroitin sulfate content in marketed products and the Caco-2 permeability of chondroitin sulfate raw materials Archived from the original on August 9, 2017. In: J Am Nutr Assoc . 3, 2000, pp. 37-44. Retrieved April 4, 2018.
- ↑ McAlindon TE, LaValley MP, Gulin JP, Felson DT: Glucosamine and Chondroitin for Treatment of Osteoarthritis: A Systematic Quality Assessment and Meta-analysis . In: Journal of the American Medical Association . 283, 2000, pp. 1469-1475. PMID 10732937 .
- ↑ Leeb BF, Schweitzer H, Montag K, Smolen JS .: A metaanalysis of chondroitin sulfate in the treatment of osteoarthritis. . In: J Rheumatol . 27, 2000, pp. 205-211 .. PMID 10648040 .
- ↑ Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, Bradley JD, Bingham CO 3rd, Weisman MH, Jackson CG, Lane NE, Cush JJ, Moreland LW, Schumacher HR Jr, Oddis CV, Wolfe F, Molitor JA, Yocum DE, Schnitzer TJ, Furst DE, Sawitzke AD, Shi H, Brandt KD, Moskowitz RW, Williams HJ: Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis . In: New Engl J Med . 354, No. 8, 2006, pp. 795-808. PMID 16495392 .
- ^ S. Reichenbach, R. Sterchi, M. Scherer, S. Trelle, E. Bürgi, U. Bürgi, PA Dieppe, P. Jüni: Meta-analysis: chondroitin for osteoarthritis of the knee or hip. In: Annals of Internal Medicine . Volume 146, Number 8, April 2007, pp. 580-590, PMID 17438317 .
- ↑ S. Wandel, P. Jüni, B. Tendal, E. Nüesch, PM Villiger, NJ Welton, S. Reichenbach, S. Trelle: Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta -analysis. In: BMJ (Clinical research ed.). Volume 341, 2010, p. C4675, PMID 20847017 . PMC 2941572 (free full text).
- ↑ M. Fransen, M. Agaliotis, L. Nairn, M. Votrubec, L. Bridgett, S. Su, S. Jan, L. March, J. Edmonds, R. Norton, M. Woodward, R. Day: Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomized placebo-controlled clinical trial evaluating single and combination regimens. In: Ann. Rheumatism. Dis. Volume 74, number 5, May 2015, pp. 851-858, doi : 10.1136 / annrheumdis-2013-203954 , PMID 24395557 .
- ↑ Jean-Pierre Pelletier, Patrick du Souich, Allen Sawitzke, Pascal Richette, Thomas Pap: Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomized, double-blind, non-inferiority trial versus celecoxib . In: Annals of the Rheumatic Diseases . tape 75 , no. 1 , January 1, 2016, p. 37-44 , doi : 10.1136 / annrheumdis-2014-206792 , PMID 25589511 ( bmj.com [accessed March 15, 2019]).
- ↑ arznei-telegramm , 7th edition, 1992, p. 66 full text (PDF; 14 kB).
- ^ S. Alban: Lessons from the heparin scandal , Pharmazeutische Zeitung , Issue 1, 2010.