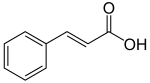
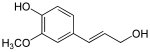
Phenylpropanoids

Phenylpropanoids ( Greek : ending -ειδἠς ( -eides ) “similar”) are organic compounds that are derived from phenylpropane , a typical carbon skeleton made up of a benzene ring and a side chain of three carbon atoms . They often have both hydroxyl and methoxy groups as substituents on the aromatic . The side chain is also very varied. There can be C = C double bonds there; often the terminal carbon atom is oxidized to alcohol , aldehyde or a carboxylic acid. Many phenylpropanoids are natural substances that are formed in plants and microorganisms via the shikimate biosynthetic pathway with phenylalanine as an intermediate. Besides the terpenes , phenylpropanoids are a common component of essential oils and represent the majority of naturally occurring phenolic natural substances or their precursors. The well-known representatives from the group of phenylpropanoids include: anethole , apiol , cinnamaldehyde , dillapiol and estragole .
Occurrence
Phenylpropanoids are a very common class of secondary plant substances , which represent the main amount of naturally occurring phenols and aromatics or are their biosynthetic precursors. The structural spectrum is very broad and they fulfill a variety of biological tasks.
An example of the biological function are the flavonoids , which protect many species against ultraviolet radiation and flower pigments. Or the biopolymer lignin , which gives the plant tissue stability.
Cinnamic acids
A central intermediate product of most phenylpropanoids is cinnamic acid or various hydroxy or methoxycinnamic acids. In many plants, however, they represent an end product in the form of conjugates. The occurrence of certain compounds is often limited to a certain number of species.
The cinnamic acids are biosynthetically produced from phenylalanine with the help of the enzyme phenylalanine ammonia lyase (PAL). A series of enzymatic hydroxylations and methylations then lead to the cinnamic acid derivatives such as p-coumaric acid , caffeic acid , ferulic acid , 5-hydroxyferulic acid and 4-hydroxy-3,5-dimethoxycinnamic acid. The esters of these cinnamic acids are volatile compounds with a tangy or floral scent and are used to attract pollinating insects. An example of this is ethyl cinnamate.
Cinnamon aldehydes and monolignols
The reduction of the carboxylic acid group in the cinnamic acids initially leads to the corresponding aldehydes such as cinnamaldehyde . A further reduction step then produces the corresponding alcohol such as coumaryl alcohol or coniferyl alcohol, which are called monolignols . These monolignols can polymerize to lignins or suberins, which are important as cell walls of plants.
The phenylpropenes such as eugenol , safrole , myristicin , elemicin , estragole are derivatives of monolignols and are components of essential oils .
Coumarins and flavonoids
The hydroxylation of cinnamic acid by the enzyme cinnamic acid 4-hydroxylase gives p- cumaric acid which, by further hydroxylation in the 2-position and subsequent cyclization, gives umbelliferone .
Flavonoids are also made from p -umaric acid. With the help of the chalcone synthase, the cumaryl-CoA reacts with three molecules of malonyl-CoA to form the chalcone . An isomerase then converts this into flavanone, from which all flavonoids are biosynthesized.
Stilbenoids
Stilbenoids such as resveratrol are hydroxylated derivatives of stilbene . They are formed by an alternative cyclization of cinnamic acid-CoA or cumaryl-CoA.
Lignans
Dimeric phenylpropanoids are known as lignans or neolignans . Lignans are dimerized via the β atom of the propyl side chain (e.g. in podophyllotoxin ), neolignans via two other atoms, e.g. B. via an atom of the propyl chain and an atom of the aromatic (3,8'-linkage) or via two aromatic atoms (z. B. 3,3'-linkage, z. B. in Honokiol ).
literature
- G. Michal (Ed.): Biochemical Pathways - Biochemie-Atlas , 1st edition, Spektrum Akademischer Verlag, Heidelberg Berlin 1999, ISBN 3-86025-239-9 .
- Peter Nuhn : Natural Products Chemistry: Microbial, Plant and Animal Natural Products , 2., neubearb. u. exp. A., Hirzel Verlag, Stuttgart 1997.
Individual evidence
- ↑ Phenylpropanoid biosynthesis - Reference pathway
- ^ The Douglas Laboratory, Department of Botany, UNIVERSITY OF BRITISH COLUMBIA: Overview of Phenylpropanoid Metabolism ( Memento June 10, 2007 in the Internet Archive ).
- ↑ D. Strack: In: PM Dey, JB Harborne, editors. Plant biochemistry; Academic Press San Diego California, USA 1997. p 387-416.
- ↑ G. Richter: In metabolic physiology of plants. 6th ed .: Thieme Verlag, Stuttgart 1998, Chapter 8, Phenols; Pp. 365-390.
- ↑ LG Landry, CCS Chapple, R. Last: Arabidopsis Mutants Lacking Phenolic Sunscreens Exhibit Enhanced Ultraviolet-B Injury and Oxidative Damage. ; Plant Physiol , 1995 , 109 , pp. 1159-1166; doi: 10.1104 / pp.109.4.1159 .
- ↑ CF Douglas: Phenylpropanoid metabolism and lignin biosynthesis: from weeds to trees. Trends Plant Sci , 1996 , 1 , pp. 171-178; doi: 10.1016 / 1360-1385 (96) 10019-4 .
- ↑ D. Strack, HP Mock: In PM Dey, JB Harborne, editors. Volume 9, methods in plant biochemistry: enzymes of secondary metabolism; Academic Press, San Diego California, USA 1993, pp. 45-97.