Safrole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
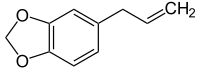
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Safrole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 10 O 2 | ||||||||||||||||||
| Brief description |
yellow liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 162.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.10 g cm −3 |
||||||||||||||||||
| Melting point |
11.2 ° C |
||||||||||||||||||
| boiling point |
234.5 ° C |
||||||||||||||||||
| Vapor pressure |
1.3 h Pa (65 ° C) |
||||||||||||||||||
| solubility |
practically insoluble in water |
||||||||||||||||||
| Refractive index |
1.5381 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Safrole is a phenylpropanoid with an anise- like odor.
Occurrence
Safrole is found in some, especially tropical, plants. It is the main component of some essential oils ; For example, in the North American sassafras oil from the sassafras tree ( Sassafras albidum ) it forms up to 90% of the volatile fraction . Other plants rich in saffron are the Central American pepper plant Makulan ( Piper auritum ) and Ocotea odorifera , Ocotea sassafras , Ocotea cymbarum and Nectandra sanguinea from the laurel family or Cinnamomum species from Asia. The most important source is the camphor tree native to Asia , the root oil of which is mainly safrole. In smaller quantities, safrole is found in a large number of plants, e.g. B. in black pepper or in nutmegs .
use
Because of its pleasant smell, plant extracts containing safrole were previously used in food preparation as well as in perfumery or as an oil for fragrance lamps. This is how the American root beer owes its typical taste to it. However, safrole is highly toxic to the liver and kidneys and is also suspected of having carcinogenic effects. Today, Safrol is not an approved food additive in any EU country . In June 2001 the Federal Institute for Consumer Health Protection and Veterinary Medicine issued a statement on the health assessment of Safrol. The use of safrole in tobacco products is prohibited by the Tobacco Ordinance.
The industrial extraction takes place via steam distillation of the root wood of the sassafras tree.
Safrole is used as the starting material for the synthesis of the insecticide synergist piperonyl butoxide .
Since it could also serve as a raw material for the synthesis of MDMA (“Ecstasy”), MDA and MDEA (“Eve”), production, acquisition, distribution or the like without authorization are now prohibited in the European Union and are prosecuted. The same goes for sassafras oil.
Individual evidence
- ↑ a b c d e f g h Entry on Safrol in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-458.
- ↑ Entry on 5-allyl-1,3-benzodioxole in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on Safrol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 1230.
- ↑ Entry on Safrol. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ BgVV: Health assessment of scented oils containing safrole, methyleugenol or estragole . BgVV statement of May 11, 2001. PDF .
literature
- Shulgin, Alexander (1967): Psychotropic Phenylisopropylamines derived from Apiole and Dillapiole. In: Nature . Volume 215, pp. 1494-1495, PMID 4861200 ( HTML ).


