Cinnamaldehyde
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of trans -cinnamaldehyde | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cinnamaldehyde | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 8 O | ||||||||||||||||||
| Brief description |
yellowish, oily liquid with an intense smell of cinnamon |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 132.16 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.05 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−8 ° C |
||||||||||||||||||
| boiling point |
246-253 ° C |
||||||||||||||||||
| Vapor pressure |
3.85 Pa (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.6219 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Cinnamaldehyde ( 3-phenyl-2-propenal , FEMA 2286 ) is a yellowish , oily liquid that smells intensely of cinnamon . It is the main flavoring of the cinnamon bark and was first isolated from cinnamon oil in 1834 . Cinnamaldehyde is an organic chemical compound with the empirical formula C 9 H 8 O and belongs to the group of phenylpropanoids . It belongs to the aromatic group and is an α, β-unsaturated aldehyde .
Isomerism
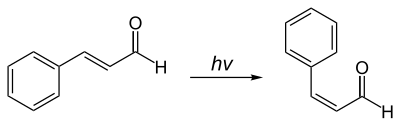
The carbon-carbon double bond in the side chain of cinnamaldehyde is typically trans -configured. The isomeric cis -cinnamic aldehyde [synonym: ( Z ) -cinnamic aldehyde] is of little importance. The information in this article relates only to trans -cinnamaldehyde [synonym: ( E ) -cinnamaldehyde].
| Isomers of cinnamaldehyde | ||
| Surname | trans -cinnamaldehyde | cis -cinnamaldehyde |
| other names | ( E ) -cinnamaldehyde | ( Z ) -cinnamaldehyde |
| Structural formula | 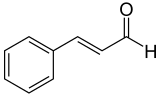 |

|
| CAS number | 14371-10-9 | 57194-69-1 |
| 104-55-2 (unspec.) | ||
| EC number | 604-377-8 | - |
| 203-213-9 (unspec.) | ||
| ECHA info card | 100.111.079 | - |
| 100.002.922 (unspec.) | ||
| PubChem | 637511 | 6428995 |
| 307 (unspec.) | ||
| Wikidata | Q204036 | Q27161918 |
| Q60041699 (unspec.) | ||
History and occurrence
Cinnamaldehyde was isolated from cinnamon oil by Jean-Baptiste Dumas and Eugène-Melchior Péligot in 1834 and synthesized by Luigi Chiozza in 1856 .
Cinnamaldehyde occurs naturally in cinnamon bark oil (42–68%) and cassia oil (up to 90%), but to a lesser extent also in the leaves of the cinnamon tree and in many other essential oils.
Cinnamon oil is extracted from the bark of the cinnamon tree ( Cinnamomum verum ), 65–75% of which is cinnamaldehyde.
presentation
Natural sources
Cinnamaldehyde is obtained from the bark (and, to a lesser extent, the leaves) of the cinnamon tree by steam distillation .
Technical syntheses
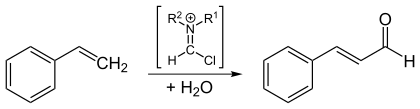
Trans- cinnamaldehyde can be prepared in a 60% yield analogous to cinnamic acid by means of aldol condensation from benzaldehyde and acetaldehyde .
The preparation is also possible by Vilsmeier formylation of styrene in 30% yield.
The cis -cinnamaldehyde can be obtained from the trans -compound by a photochemical isomerization :
properties
Physical Properties
Cinnamaldehyde is a yellowish, oily liquid that smells intensely of cinnamon and which gradually oxidizes to cinnamic acid in the air . It melts at −8 ° C and boils at 251 ° C at normal pressure . It dissolves very poorly in water (1.1 g / l at 20 ° C), but it is miscible with ethanol , diethyl ether and chloroform .
Chemical properties
Cinnamaldehyde is sensitive to light, heat, alkalis and some metals and shows the usual aldehyde and olefin reactions.
It can be reduced to cinnamon alcohol in 75% yield by Meerwein-Ponndorf-Verley reduction .
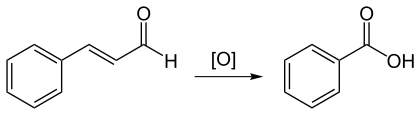
Cinnamic acid is formed through autoxidation in air .
Stronger oxidizing agents such as potassium permanganate or ozone oxidize cinnamaldehyde to benzoic acid.
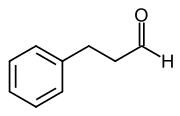
Hydrocinnamaldehyde is formed by hydrogenation of the double bond .
Structural relatives
One derivative is coniferylaldehyde [ 3- (4-hydroxy-3-methoxyphenyl) -2-propenal ], which on the benzene ring also carries a hydroxy group at the 4-position and a methoxy group at the 3-position , and thus the substitution pattern as in the case of vanillin .
 |
 |

|
| Cinnamon alcohol | Cinnamaldehyde | Cinnamic acid |
use
Cinnamaldehyde is used as a fragrance in perfume production in the composition of oriental perfumes. It is also used as a fragrance in cosmetics, e.g. B. in lipsticks, detergents, cleaning agents, toothpaste and mouthwash. It is also used as a spice in food.
In its pure form it has sensitizing properties and can lead to allergic skin reactions. Patients with a cinnamaldehyde allergy also often react to balsam of Peru , which indicates cross-allergies . The compound reacts with nucleophilic structural components of proteins , especially the thiol groups of cysteine , and can thus trigger a delayed-type immune reaction. Due to its high allergenic potential , it is part of the so-called "fragrance mix" for patch tests .
Web links
- Entry to 2-propenal, 3-phenyl- . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed September 10, 2013.
- Fragrance Lexicon: Cinnamaldehyde
Individual evidence
- ↑ a b Entry on FEMA 2286 in the database of the Flavor and Extract Manufacturers Association of the United States .
- ↑ a b c d e f g h i j k l Entry on cinnamaldehyde in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b c Entry on cinnamaldehyde. In: Römpp Online . Georg Thieme Verlag, accessed on April 19, 2014.
- ↑ a b data sheet trans-cinnamaldehyde (PDF) from Merck , accessed on September 10, 2013.
- ↑ a b c d Committee for Hazardous Substances: Explanation on cinnamaldehyde in TRGS 907 - Edition: December 2011 - Status: May 2011 .
- ↑ a b Dieter Martinetz, Roland Hartwig: Pocket book of fragrances: a lexicon from AZ . Harri Deutsch Verlag, 1998, ISBN 3-8171-1539-3 , p. 404 ( limited preview in Google Book Search).
- ^ Albert Gossauer: Structure and Reactivity of Biomolecules , Verlag Helvetica Chimica Acta, Zurich 2006, ISBN 978-3-906390-29-1 , p. 313.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 470.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 345.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 505.
- ↑ Patent US3372199 : Method for the production of Process for the production of hydrocinnamaldehyde . Applied March 20, 1964 , published March 5, 1968 , Applicant: Engelhard Corporation , Inventor: Nathan Himselstein, Paul N. Rylander .
- ↑ Juliane Daphi-Weber, Heike Raddatz, Rainer Müller: Investigation of Fragrances - Controlled Fragrances , pp. 94–95, in Volume V of the series HighChem hautnah - News from food chemistry (published by the Society of German Chemists ) 2010, ISBN 978- 3-936028-64-5 .
- ^ Jean-Marie Lachapelle: Patch Testing and Prick Testing. Springer Science & Business Media, 2012, ISBN 978-3-642-25492-5 , p. 108 ( limited preview in Google book search).
- ↑ Axel Trautmann: Allergy Diagnosis Allergy Therapy. Thieme, 2006, ISBN 978-3-13-169061-6 ( limited preview in Google book search).