Cinnamon alcohol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
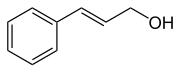
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cinnamon alcohol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 10 O | ||||||||||||||||||
| Brief description |
sweet-balsamic hyacinth-scented needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 134.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.04 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
31-34 ° C |
||||||||||||||||||
| boiling point |
258 ° C |
||||||||||||||||||
| Vapor pressure |
1.33 hPa (114 ° C) |
||||||||||||||||||
| solubility |
poor in water (1.8 g l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Cinnamyl alcohol ( 3-phenyl-2-propenol , FEMA 2294 ), also known as Styron referred to is a naturally occurring perfume , in the cosmetics industry is used. Cinnamon alcohol is an aromatic compound and is an unsaturated alcohol with a trans -substituted carbon-carbon double bond in the side chain. The isomeric cis or ( Z ) cinnamon alcohol is of little importance. The information in this article relates only to the trans cinnamon alcohol.
Occurrence
Cinnamon alcohol is found in a wide variety of plants. It is included in:
Extraction and presentation
Cinnamon alcohol can be obtained directly from cinnamon or from cinnamaldehyde by the Meerwein-Ponndorf-Verley reduction in 75% yield.
It is also possible to convert phenylpropargyl alcohol to cinnamon alcohol with the aid of lithium aluminum hydride .
properties
Physical Properties
Cinnamon alcohol forms sweet-balsamic needles that smell of hyacinths. It is poorly soluble in water, readily soluble in diethyl ether and miscible with ethanol .
Chemical properties
Cinnamon alcohol can be oxidized with manganese dioxide at room temperature in ether to form cinnamaldehyde in 87% yield.
use
Cinnamon alcohol is used as an additive (fragrance) in cosmetics (e.g. massage and baby oils, toilet paper) to obtain a violet or hyacinth scent. It is also used to make derivative compounds such as hydrocinnamon alcohol (phenylpropanol).
safety instructions
Cinnamon alcohol is a naturally occurring allergen .
See also
Web links
Individual evidence
- ↑ a b Entry on FEMA 2294 in the database of the Flavor and Extract Manufacturers Association of the United States .
- ↑ Entry on cinnamon alcohol. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ Entry on 2-propen-1-ol, 3-phenyl- . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed September 24, 2012.
- ↑ a b c d e f g h i j Entry on cinnamon alcohol in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-432.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 505.
- ^ Gattermann / Wieland : The practice of organic chemists , 43rd edition, Walter de Gruyter, Berlin · New York 1982, ISBN 3-11-006654-8 , pp. 483-484: Brownstone oxidation of cinnamon alcohol .
- ↑ Juliane Daphi-Weber, Heike Raddatz, Rainer Müller: Investigation of Fragrances - Controlled Fragrances , pp. 94–95, in Volume V of the series HighChem hautnah - News from food chemistry (published by the Society of German Chemists ) 2010, ISBN 978- 3-936028-64-5 .
- ↑ K. Jason Dennis and Takayuki Shibamoto: Photochemical Products of trans-Cinnamic Alcohol: Possible Formation of Skin Irritants and Allergens , In: J. Toxicol. Cutan. Ocul. Toxicol. , 1990 , 9 (2), pp. 149-157 ( doi : 10.3109 / 15569529009036319 ).
- ^ Heinrich Dickel: Implementation of a relational database system in the University Dermatology Clinic in Cologne with the evaluation of a bicontinental multicenter study - BAER study - on the question of allergen frequency in contact allergies . Dissertation, TH Aachen, 1996. DNB 97070903x / 34 .



