Apiol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
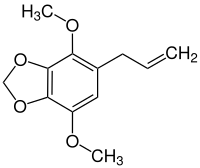
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Apiol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 14 O 4 | ||||||||||||||||||
| Brief description |
colorless, needle-shaped crystals with a characteristic smell of parsley |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 222.23 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.02 g cm −3 |
||||||||||||||||||
| Melting point |
29.5 ° C |
||||||||||||||||||
| boiling point |
294 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Apiol is a phenylpropanoid and an integral part of the essential oils of various types of parsley and celery . Apiol is an isomer of Dillapiol .
Already in the Middle Ages, preparations containing apiol were used as herbal remedies for termination of pregnancy .
In 1715, the Leipzig pharmacist Heinrich Christoph Link discovered Apiol while steam distilling parsley oil. In 1855, Joret and Homolle found that treatment with Apiol was very effective in treating amenorrhea (menstrual cramps).
Apiol can cause allergic reactions. High doses lead to liver and kidney damage.
Individual evidence
- ↑ a b c d Entry on Apiol. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ a b c Entry on Apiol in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on November 28, 2018.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ André Patoir et al. a .: Étude expérimentale compartive de quelques abortifs (Apiol, Rue, Sabine, Armoise). In: Gynéc. et Obstétr. Volume 39, 1939, pp. 201-209.
- ^ Museum of Contraception and Abortion: Parsley
literature
- Shulgin, Alexander (1967): Psychotropic Phenylisopropylamines derived from Apiole and Dillapiole. In: Nature . Vol. 215, pp. 1494-1495. PMID 4861200 , HTML
