Vanillyl alcohol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
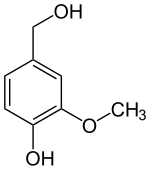
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Vanillyl alcohol | |||||||||||||||||||||
| other names |
4-hydroxy-3-methoxybenzyl alcohol |
|||||||||||||||||||||
| Molecular formula | C 8 H 10 O 3 | |||||||||||||||||||||
| Brief description |
white or colorless crystals with a mild, sweet, vanilla-like odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 154.17 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
114-115 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Vanillyl alcohol ( 4-hydroxy-3-methoxybenzyl alcohol ) is an organic chemical compound naturally found in Sitka spruce ( Picea sitchensis ). The substance is structurally derived from both benzyl alcohol and guaiacol ( o -methoxyphenol). The white or colorless crystals with a mild, sweet, vanilla-like odor dissolve only slightly in water, but dissolve well in oils.
A catalytic hydrogenation of vanillin leads to vanillyl alcohol or 2-methoxy-4-methylphenol .
The vanillyl-alcohol oxidase catalyzes the oxidation of various phenolic compounds, especially many 4-alkylphenols. The oxidation of vanillyl alcohol to vanillin was originally demonstrated in vitro :
Vanillyl alcohol is classified as FEMA GRAS ( G enerally R ecognized A s S afe , FEMA GRAS # 3737) in the United States and can therefore be used there as a food additive .
See also
Individual evidence
- ↑ a b c Shmuel Yannai: Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients. CRC Press , 2003, ISBN 978-1-58488-416-3 , p. 307.
- ↑ a b D. C. Ayres (Ed.): Dictionary of Natural Products, Volume 6. CRC Press , 1994, ISBN 978-0-412-46620-5 , p. 1562.
- ↑ Entry on vanillyl alcohol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Data sheet Vanillyl alcohol from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ↑ Fragrance Lexicon: Vanillin .
- ↑ A. Mattevi, MW Fraaije, A. Mozzarelli, L. Olivi, A. Coda, WJ van Berkel: Crystal structures and inhibitor binding in the octameric flavoenzymes vanillyl-alcohol oxidase: the shape of the active-site cavity controls substrates specificity , in: Structure , 1997 , 5 (7), pp. 907-920 ( PMID 9261083 ; full text ).
- ↑ George A. Burdock: Encyclopedia of food and color additives, Volume 3. CRC Press , 1997, ISBN 978-0-8493-9414-0 , p. 2903.
Web links
- Entry on vanillyl alcohol . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed December 14, 2012.