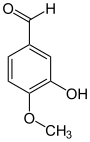
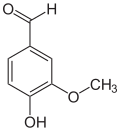
Isovanillin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Isovanillin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 8 O 3 | ||||||||||||||||||
| Brief description |
pale yellowish, glass-glossy columns |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 152.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
113-115 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| pK s value |
8.89 (25 ° C) |
||||||||||||||||||
| solubility |
Easily soluble in hot water, hardly soluble in cold water; very easily soluble in chloroform , easily in ethanol , ether , methanol and glacial acetic acid |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Isovanillin ( 3-hydroxy-4-methoxybenzaldehyde ) is an organic chemical compound with the empirical formula C 8 H 8 O 3 . It is a derivative of benzaldehyde with an additional hydroxyl and methoxy group . Isovanillin is an almost odorless isomer of vanillin , from which it differs only by the position of the methoxy group. It serves as a synthesis component and is mainly used in the pharmaceutical, cosmetic, agricultural and food sectors.
History and occurrence
Isovanillin was isolated and characterized by Rudolf Wegscheider in 1882 . It comes u. a. in Mondia whitei and cassava .
Extraction and presentation
Natural sources
Isovanillin is isolated either by extraction with various solvents or by steam distillation from dried roots of Mondia whitei .
Technical syntheses
Numerous methods are known for the representation:
- Piperonal ( 3,4- (methylenedioxy) benzaldehyde ) is reacted with sodium methoxide in the presence of copper (I) chloride in dimethylformamide .
- Veratrumaldehyde ( 3,4-dimethoxybenzaldehyde ) can be converted to isovanillin by selective demethylation with methionine in methanesulfonic acid . The reaction times are long and the selectivity is not very high.
- Selective O-methylation of protocatechualdehyde ( 3,4-dihydroxybenzaldehyde ) by methyl iodide in the presence of sodium hydride in dimethyl sulfoxide leads to a yield of about 65% isovanillin.
- A formylation of guaiacol ( o-methoxyphenol ), which is protected as acetate, takes place with dichloromethoxymethane in the presence of titanium tetrachloride in dichloromethane . The 3-acetoxy-4-methoxybenzaldehyde is then hydrolyzed with NaOH .
- Isovanillin can be obtained in good yield by using a strong acid to selectively split off the alkyl group in the 3-position of a 3-alkoxy-4-methoxybenzaldehyde, the alkoxy group containing at least two carbon atoms. This is u. a. Ethylvanillin ( 1 ), which is methylated with dimethyl sulfate to give 3-ethoxy-4-methoxybenzaldehyde ( 2 ). The ethyl ether is then selectively cleaved with sulfuric acid .
properties
Physical Properties
Isovanillin forms pale yellowish, glass-glossy columns, melts at 113–115 ° C and boils at 179 ° C under reduced pressure (15 mmHg ; ≈ 20 hPa ). It is easily soluble in hot water, but hardly soluble in cold water; however, it is easily soluble in chloroform , ethanol , ether , methanol and glacial acetic acid . It crystallizes in the monoclinic crystal system in the space group P 2 1 / a (space group no. 14, position 3) with the lattice parameters a = 851.7 pm , b = 1338.0 pm, c = 639.0 pm, β = 97 , 21 ° and four formula units per unit cell .
Chemical properties
Isovanillin is structurally derived from both benzaldehyde and guaiacol ( 2-methoxyphenol ). As an isomer, it differs from vanillin in the position of the methoxy group. Instead of position 3, this is found here in position 4. Hydroxy and methoxy groups swap places compared to vanillin. In contrast to vanillin, it is almost odorless.
However, it does not form a color with an aqueous solution of iron (III) chloride . The pK s value of the phenolic OH group is 8.89 (25 ° C). This value is lower than the phenol at 9.99; the electron-withdrawing aldehyde group increases the OH acidity through its −M effect ; the phenolic OH bond is increasingly polarized.
Biological properties
Isovanillin is a selective inhibitor of aldehyde oxidase . It is not a substrate for this enzyme and is metabolized to isovanillic acid by aldehyde dehydrogenase .
Structural relatives
Isovanillyl alcohol ( 3-hydroxy-4-methoxybenzyl alcohol ) is produced by reducing isovanillin. Isovanillic acid ( 3-hydroxy-4-methoxybenzoic acid ) is produced by oxidation, e.g. B. by enzymatic route.
Isoethylvanillin ( 3-hydroxy-4- eth oxybenzaldehyde ) is a structural relatives and differs from isovanillin by replacing the methyl group to ethyl. The structural analogy corresponds to that between vanillin and ethylvanillin .
iso- acetovanillon ( 3-hydroxy-4-methoxyacetophenone ) is also structurally related and differs from isovanillin in that the formyl group ( aldehyde group ) is exchanged for an acetyl group . The structural analogy corresponds to that between vanillin and acetovanillon .
use
Isovanillin is used as a synthesis building block, especially in the pharmaceutical, cosmetic, agricultural and food sectors.
Individual evidence
- ↑ a b c d e f Rudolf Wegscheider: "About Isovanillin", in: Monatshefte für Chemie , 1882 , 3 (1), pp. 789–795 ( doi : 10.1007 / BF01516846 ).
- ↑ a b c d e f data sheet 3-Hydroxy-4-methoxybenzaldehyde from Sigma-Aldrich , accessed on January 31, 2013 ( PDF ).
- ↑ a b c CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 , p. 434, no. 54.
- ↑ a b Neil A. Koorbanally, Dulcie A. Mulholland, Neil R. Crouch: "Isolation of isovanillin from Aromatic Roots of the Medicinal African Liane, Mondia whitei ", in: Journal of Herbs, Spices & Medicinal Plants , 2000 , 7 ( 3), pp. 37-43 ( doi : 10.1300 / J044v07n03_05 ).
- ↑ a b Kavaka W. Mukonyi, Isaiah O. Ndiege: "2-Hydroxy-4-methoxybenzaldehyde: Aromatic Taste Modifying Compound from Mondia whytei Skeels ", in: Bull. Chem. Soc. Ethiop. , 2001 , 15 (2), pp. 137-141 ( PDF ).
- ↑ Bo Yi, Lifei Hu, Wenli Mei, Kaibing Zhou, Hui Wang, Ying Luo, Xiaoyi Wei, Haofu Dai: "Antioxidant Phenolic Compounds of Cassava ( Manihot esculenta ) from Hainan", in: Molecules , 2010 , 16 , p. 10157 -10167 ( doi : 10.3390 / molecules161210157 ; PDF ).
- ↑ Patent DE102009046126: Orally consumable preparation comprising root extract from Mondia whitei .
- ↑ a b c Patent specifications: PatentDE: Process for the production of isovanillin ; United States Patent 5648552: Process for the preparation of isovanillin .
- ↑ N. Yu. Baratov, VI Vinogradova, MS Yunusov (Inst. Khim. Rastit. Veshchestv, Tashkent, USSR): "Reaction of 3,4-methylenedioxybenzaldehydes with sodium methoxide", in: Zh. Org. Khim. , 1991 , 27 (7), pp. 1578-1579.
- ↑ Nobutaka Fujii, Hiroshi Irie, Haruaki Yajima: "Regioselective Cleavage of Aromatic Methyl Ethers by Methanesulphonic Acid in the Presence of Methionine", in: J. Chem. Soc., Perkin Trans. 1 , 1977 , 20 , pp. 2288-2288 ( doi : 10.1039 / P19770002288 ).
- ↑ Satinder V. Kessar, Yash P. Gupta, Taj Mohammed, (Miss) Manju Goyal, Kewal K. Sawal: "Regioselective Mono- O -alkylation of some Pyrocatechoxide Dianions", in: J. Chem. Soc., Chem. Commun . , 1983 , 7 , pp. 400-401 ( doi : 10.1039 / C39830000400 ).
- ↑ Maria Luisa Scarpati, Armandodoriano Bianco, Livia Mascitelli, Pietro Passacantilli: "Selective Formylation of diphenol," in Synth. Commun. , 1990 , 20 (17), pp. 2565-2572 ( doi : 10.1080 / 00397919008051462 ).
- ↑ Fujiko Iwasaki: "The Crystal Structure of 3-Hydroxy-4-methoxybenzaldehyde (Isovanillin)", in: Chemistry Letters , 1973 , 2 (3), pp. 227-228 ( doi : 10.1246 / cl.1973.227 ; PDF ).
- ↑ Toru Egawa, Akiyo Kameyama, Hiroshi Takeuchi: "Structural determination of vanillin, isovanillin and ethylvanillin by means of gas electron diffraction and theoretical calculations", in: Journal of Molecular Structure , 2006 , 794 (1-3), p. 92– 102 ( doi : 10.1016 / j.molstruc.2006.01.042 ; PDF ).
- ↑ Fragrance Lexicon: Vanillin .
- ↑ Georgios I. Panoutsopoulos, Christine Beedham: Enzymatic oxidation of phthalazines with guinea pig liver aldehyde oxidase and liver slices: inhibition by isovanillin . In: Acta Biochimica Polonica , 2004 , 51 (4), pp. 943-951 ( PMID 15625566 ; PDF ).
- ↑ Georgios I. Panoutsopoulos, Demetrios Kouretas, Christine Beedham: Contribution of aldehydes oxidase, xanthine oxidase, and aldehyde dehydrogenase on the Oxidation of Aromatic aldehyde . In: Chemical Research in Toxicology , 2004 , 17 (10), pp. 1368-1376 ( doi : 10.1021 / tx030059u ; PMID 15487898 ).
- ↑ Georgios I. Panoutsopoulos: phenylacetaldehydes oxidation by freshly prepared and cryopreserved guinea pig liver slices: the role of aldehyde oxidase . In: International Journal of Toxicology , 2005 , 24 (2), pp. 103-109 ( doi : 10.1080 / 10915810590936373 ; PMID 16036769 ).
- ↑ Georgios I. Panoutsopoulos, Christine Beedham: Metabolism of isovanillin by aldehyde oxidase, xanthine oxidase, aldehyde dehydrogenase and liver slices . In: Pharmacology , 2005 , 73 (4), pp. 199-208 ( doi : 10.1159 / 000082860 ; PMID 15627845 ).
- ↑ a b Georgios I. Panoutsopoulos, Christine Beedham: "Enzymatic oxidation of vanillin, isovanillin and protocatechuic aldehyde with Freshly Prepared Guinea Pig Liver Slices", in: Cell Physiol Biochem , 2005 , 15 (1-4), pp 89-98 ( PMID 15665519 ; PDF ).
- ↑ Keng-Shiang Huang, Eng-Chi Wang: "Synthesis of Substituted Indenes from Isovanillin via Claisen Rearrangement and Ring-closing Metathesis", in: Journal of the Chinese Chemical Society , 2004 , 51 , pp. 383-391 ( doi: 10.1002 /jccs.200400060 ).
- ↑ Sie-Rong Li, Chung-Jung Shu, Liang-Yeu Chen, Hsing-Ming Chen, Po-Yuan Chen, Eng-Chi Wang: "Synthesis of substituted 2-aroyl-3-methylchromen-4-ones from isovanillin via 2 -aroyl-3-methylchroman intermediates ", in: Tetrahedron , 2009 , 65 (42), pp. 8702-8707 ( doi: 10.1016 / j.tet.2009.08.043 ).
- ↑ Roger M. Davey, N. Patrick J. Stamford: "Catalytic enamines from dialkylamide-dialkylacetals", in: Tetrahedron Letters , 2012 , 53 (20), pp. 2537-2539 ( doi: 10.1016 / j.tetlet.2012.03. 028 ).
literature
- RH Prager, YT Tan: "Selective demethylation of 3,4-dimethoxybenzaldehyde", in: Tetrahedron Letters , 1967 , 8 (38), pp. 3661-3664 ( doi : 10.1016 / S0040-4039 (01) 89768-9 ).
- V. Balachandran, K. Parimala: "Vanillin and isovanillin: Comparative vibrational spectroscopic studies, conformational stability and NLO properties by density functional theory calculations", in: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy , 2012 , 95 , p. 354– 368 ( doi : 10.1016 / j.saa.2012.03.087 ; PMID 22542395 ).
Web links
- Entry on isovanillin . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 1, 2012.
- NMRanalyst Sample Application: Isovanillin .