Ether cleavage
As ether cleavage reactions are referred to, where ethers are cleaved substitutes. Since ethers are chemically very stable, their cleavage is only possible under strongly acidic or extremely basic conditions.
In organic chemistry , ether cleavage is an acid-catalyzed nucleophilic substitution that leads to its cleavage. Depending on the ether considered, the S N 1 or S N 2 mechanism can be run through. To distinguish between the two mechanisms, it is essential to consider inductive and mesomeric effects that stabilize or destabilize a potential carbocation . The use of hydrohalic acids is advisable , as these both cause the acidic activation of the ether oxygen atom and provide the halide ion as a nucleophile . Ethers have a similar acidity, such as alcohols (pK s to 16).
In organometallic chemistry , ether cleavage refers to the decomposition of ethereal solvents by extremely basic organometallic reagents. Cyclic ethers are particularly sensitive to ether cleavage, but acyclic ethers can also be cleaved.
Ether cleavage according to the S N 1 mechanism
The unimolecular S N 1 mechanism proceeds via a carbocation, if this can be adequately stabilized. In the example, the oxygen in the tert-butyl methyl ether is reversibly protonated, with the resulting oxonium ion releasing methanol . The comparatively stable tert- butyl cation is nucleophilically attacked by a halide, here the bromide ion, so that 2-bromo-2-methylpropane is obtained.
mechanism
Ether cleavage according to the S N 2 mechanism
If the stabilization of a carbocation is not possible, the bimolecular, concerted S N 2 mechanism is run through. Here the ether oxygen in the methyl n-propyl ether is reversibly protonated, whereupon the bromide (or halide) ion attacks the sterically less hindered carbon atom with the formation of methyl bromide and 1-propanol is released at the same time .
mechanism
Other factors
As described, reactions that would cause unstable carbocations (methyl, vinyl, aryl or primary carbocations) in the S N 1 mechanism proceed via the S N 2 mechanism. An ether cleavage after S N 1 proceeds in principle faster than after S N 2. The hydrohalic acid used also plays an important role. The rate of reaction with hydriodic acid is greater than that with hydrobromic acid . If one wishes to use hydrochloric acid , more drastic reaction conditions must be used. This is due to the greater acidity of the heavier homologues of the hydrohalic acids and the greater nucleophilicity of the respective conjugated bases . The nucleophilicity of the fluoride ion is too low for hydrofluoric acid to be used for ether cleavage in the protic medium. The reaction rate is nevertheless comparatively low in any case, so that the reaction mixture has to be heated.
Ether cleavage by organometallic reagents
mechanism
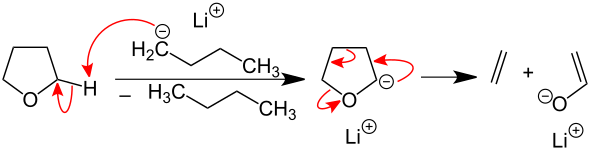
Basic ether cleavage is induced by deprotonation in the α position . The ethers then react, splitting into an alkene and an alcoholate . With cyclic ethers such as the frequently used THF , the reaction can take place in a concerted manner and is therefore particularly fast:
In the case of acyclic ethers, the deprotonation initially leads to β-hydride elimination and formation of an alkenic ether. The hydride formed then attacks the alkene radical in the α-position to the ether oxygen again and thus displaces the alcoholate.
meaning
Organometallic reagents are often handled in ethereal solvents, as these can coordinate to the metallic centers and in this way enable the organic residues to react. Here the problem of ether cleavage occurs, which not only decomposes the solvent, but also consumes the organometallic reagent. Reactions with organometallic reagents are therefore typically carried out at low temperatures ( −78 ° C ) at which the deprotonation of the ethers is kinetically inhibited .
literature
- PY Bruice: Organic Chemistry. 5th updated edition. Pearson Education, Munich 2007, ISBN 978-3-8273-7190-4 , pp. 519-520.
Individual evidence
- ↑ Christoph Elschenbroich: Organometallchemie, 5th, revised edition 2005, Wiley-VCH Weinheim. ISBN 3-519-53501-7 .