Methanesulfonic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
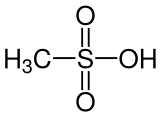
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methanesulfonic acid | |||||||||||||||
| other names |
Methyl sulfonic acid (MSS) |
|||||||||||||||
| Molecular formula | CH 4 O 3 S | |||||||||||||||
| Brief description |
colorless and odorless, viscous liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 96.11 g mol −1 | |||||||||||||||
| Physical state |
solid or liquid |
|||||||||||||||
| density |
1.48 g cm −3 |
|||||||||||||||
| Melting point |
19 ° C |
|||||||||||||||
| boiling point |
167 ° C (13 hPa ) |
|||||||||||||||
| Vapor pressure |
100 mPa (23 ° C) |
|||||||||||||||
| pK s value |
−1.9 |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Methanesulfonic acid (MSA, from English m ethane s ulfonic a cid ) is the simplest sulfonic acid . The organic salts and esters of methanesulfonic acid are referred to as methanesulfonates or mesylates or mesilates .
presentation
Methanesulfonic acid can be prepared on a laboratory scale through a number of reactions; so z. B. by oxidation of methanethiol or dimethyl disulfide with chlorine , oxygen or nitric acid . Another variant of the reaction is the reaction of sodium sulfite with dimethyl sulfate in aqueous solution at 100 ° C. and a pH greater than 6, which gives methanesulfonic acid yields of 75-85%.
The reaction of sulfur trioxide with methane is possible, but gave only low yields for a long time. The latest work describes high pressure reactions in stirred tank reactors or (semi) continuous flow reactors in the presence of free radical initiators such as dialkylsulfonyl peroxides, in particular bis (methanesulfonyl) peroxide CH 3 -SO 2 -OO-SO 2 -CH 3 (DMSP) or monomethylsulfonyl peroxides ,
in which yields of methanesulfonic acid of over 90% can be achieved.
Industrially, they are mainly produced by the oxidation of methanethiol or dimethyl disulfide with chlorine. In addition, MA has been obtained on an industrial scale for a number of years via the catalytic oxidation of dimethyl disulfide with atmospheric oxygen and nitric acid.
The conversion of methane with sulfur trioxide was made ready for large-scale production in 2016. A system for this is being planned. The start of production is expected in 2019.
properties
Methanesulfonic acid is a strong acid, its pKa value is given as −0.6 or the more likely −1.9. It can be mixed with water in any ratio and can itself serve as a solvent . The cryoscopic constant is 5.69 K. At normal pressure, MSA, which is very difficult to ignite, decomposes in an air atmosphere from about 220 ° C to carbon monoxide , carbon dioxide , sulfur trioxide and water. The vapor pressure is under standard conditions with 5.4265 × 10 -5 similarly low hPa as that of sulfuric acid . MSA does not have an oxidizing effect even in high concentrations. The high conductivity of aqueous MA solutions correlates with the acid strength, which reaches its maximum at 20–40% by weight with over 500 mS / cm and is comparable to that of inorganic acids. Due to autoprotolysis , pure MSA conducts the electrical current:
The observed proton transfer mechanism between the ions is comparable to that of other sulfonic acids . After a few days in the air, an MSA concentration of around 49% is achieved with 70–100% mixtures. Like sulfuric acid, MSA has a strong dehydrating effect and can therefore carbonize organic substances that contain hydroxyl groups . The slightly yellow-brown color of the MSA, which can occasionally be observed, is based on this property. MSA cannot be hydrolyzed by hot water or hot aqueous alkalis. The electrochemical window of the MSA is quite wide - at an applied voltage of −1.40 V there is a reduction of the acid protons, at +2.35 V there is an oxidative decomposition of the acid. It is only slightly corrosive and, even in high concentrations at temperatures around 100 ° C, cannot dissolve oxide layers of metals such as aluminum , titanium or niobium .
INN nomenclature
In the medical and pharmaceutical sector, the internationally recognized short form for the anion of methanesulphonic acid (methanesulphonate) according to the INN rules is "mesilate". Such short forms are created for molecular components if their systematic designation is too long. The “modified INN” (INNm) is created by combining a short form with the INN of the active component of the drug. One example is the drug doxazosin mesilate, derived from the nitrogenous base doxazosin .
In English there is a variant ( “mesylate” ) that corresponds to the spelling of the INN nomenclature ( “mesilate” ), which corresponds to the spelling of the United States Adopted Name Council .
use
MA serves as a solvent and catalyst for various organic reactions such as B. alkylations , esterifications , polymerizations or heterocycle syntheses . Using thionyl chloride , it can be converted into reactive methanesulfonyl chloride, with which hydroxyl groups can be converted into methanesulfonic acid esters - the methanesulfonate anion represents the significantly better leaving group in the context of nucleophilic substitutions because it is less reactive Compound, despite its strong acidity (stronger than phosphoric acid), is significantly safer than classic cleaning acids such as phosphoric, hydrochloric and sulfuric acid. The resulting alkaline earth, iron and manganese methanesulfonates are very soluble in water. Since the mercury, lead and silver methanesulfonates, in contrast to the corresponding insoluble hydrochloric and sulfuric acid salts, also dissolve very well in water, they can be used as electrolytes in methanesulfonic acid electroplating baths . Some basic drugs are used as methanesulfonic acid salts, e.g. B. rasagiline .
links
In many cases methanesulfonates can be obtained from the acid and the carbonate , hydroxide or oxide of the respective metal cation. Electrolytic production is also possible , in which the metal to be oxidized is anodically connected in an MSA bath. The methanesulfonates of various nitrogenous bases such as. B. ammonia , methylamine or 1-ethyl-3-methylimidazolium (EMIM, see 1-ethyl-3-methylimidazolium chloride , EMIM [Cl]). The reaction with EMIM results in an ionic liquid . The salts of the MA are basically very soluble in water, since the formation of a stable ion lattice is made more difficult by the monovalent nature of the anion and the non-polar methyl radical. The latter enables both the acid and the salts to dissolve to a certain extent in non-polar solvents. Most methanesulfonates are stable up to around 400 ° C, after which thermal decomposition of the organic methyl group occurs. The salts are therefore more temperature-resistant than the acid itself.
The anion has already been investigated in terms of its structural chemistry; it has approximately C 3v symmetry . It is also able to function as a complex ligand . The classification in the spectrochemical series is based on Cl - <F - ~ SO 3 Cl - ~ SO 3 F - <SO 4 2− <CH 3 SO 3 - <H 2 O, in the nephelauxetic series according to Cl - <CH 3 SO 3 - <SO 4 2− <SO 3 Cl - ~ SO 3 F - ~ H 2 O <F - . The catalytic properties of some methanesulfonates - among others as chloride-free Lewis acids - have been investigated intensively for several years; For example, lanthanide methanesulfonates achieve positive effects in some esterification reactions.
Environmental chemistry
MSA is an indicator of the amount of sulfur emissions of oceanic origin that enter the atmosphere. It is therefore also an indicator of the acidity of the atmosphere. There, MSA is created as an intermediate product of the natural sulfur cycle during the photochemical conversion of dimethyl sulfide to sulfate aerosols .
According to OECD guideline 301 A, MSA is easily biodegradable, with carbon dioxide and sulfate being produced as degradation products.
Web links
Individual evidence
- ↑ Data sheet methanesulfonic acid (PDF) from Merck , accessed on January 18, 2011.
- ↑ a b c Methanesulfonic acid . In: The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals . 14th edition. Merck & Co., Whitehouse Station NJ 2006, ISBN 978-0-911910-00-1 , p. 698.
- ↑ a b c d e f g h Lutropur - the friendly acid. BASF , accessed on September 21, 2017 (PDF; 707 kB).
- ↑ YR Clarke, LA Woodward, Trans. Faraday Soc. 1966, 62, 2226.
- ^ AK Covington, R. Thompson, Journal of Solution Chemistry 1974, 3, 603-617.
- ↑ a b entry to methane in the GESTIS Bank of IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Methanesulphonic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet methanesulfonic acid 70%, for synthesis (PDF) from Carl Roth , accessed on May 30, 2014.
- ↑ Patent US6060621 : Process for the preparation of methanesulfonic acid. Registered on October 1, 1998 , published on January 9, 2000 , applicant: Grillo-Werke AG, inventors: I. Biertümpel, K. Driemel, J. van de Flierdt, DMM Rohe.
- ↑ Patent US2493038 : Reaction of methane with sulfur trioxide. Applied on May 31, 1946 , published January 3, 1950 , applicant: Houdry Process Corporation, inventor: Aristid V. Grosse, John C. Snyder.
- ↑ LJ Lobree, AT Bell: K 2 S 2 O 8 -Initiated Sulfonation of Methane to Methanesulfonic Acid . In: Industrial & Engineering Chemistry Research . tape 40 , no. 3 , 2001, p. 736-742 , doi : 10.1021 / ie000725b .
- ^ A b Michael McCoy: German firm claims new route to methanesulfonic acid | June 27, 2016 Issue - Vol. 94 Issue 26 | Chemical & Engineering News. In: cen.acs.org. Retrieved July 4, 2016 .
- ↑ Patent US20160289176A1 : Process for preparing alkanesulfonic acids from sulfur trioxide and an alkane. Registered on November 13, 2014 , published on October 6, 2016 , applicant: Grillo Chemie GmbH, inventor: T. Ott, I. Biertümpel, K. Bunthoff, A. Richards.
- ↑ Patent US261907 : Di (methanesulfonyl) peroxide and its preparation. Registered January 5, 1951 , published November 25, 1952 , Applicant: The Dow Chemical Co., Inventor: GD Jones, RE Friedrich.
- ↑ Patent US20160289181A1 : Novel initiator for preparing alkanesulfonic acids from alkane and oleum. Registered on November 17, 2014 , published on October 6, 2016 , applicant: Grillo-Werke AG, inventor: T. Ott, I. Biertümpel.
- ↑ a b M. D. Gernon, M. Wu, T. Buszta, P. Janney, In: Green Chemistry 1999, 127-140.
- ↑ Patent EP1133470B1 : Process for the production of alkanesulfonic acids. Registered on November 22, 1999 , published on May 21, 2003 , applicant: BASF AG, inventor: Klaus Ebel, Matthias Eiermann, Christian Tragut.
- ↑ Patent EP1133472B1 : Process for the production of organic disulfides. Registered on November 25, 1999 , published on February 23, 2005 , applicant: BASF AG, inventor: Werner Hesse, Hans-Josef Sterzel, Christian Tragut.
- ↑ S. Brownstein, AE Stillman, J. Phys. Chem. 1959, 63, 2061-2062.
- ^ RC Paul, KK Paul, KC Malhotra, J. Chem. Soc. (A) 1970, 2712-2715.
- ^ RA Craig, AG Garrett, SM Newman, J. Am. Chem. Soc. 1950, 72, 163-166.
- ↑ a b c BASF SE, EMV 0101 e 2008, New applications involving methanesulfonic acid.
- ↑ R. Ch. Paul, VP Kapila, R. Kuma, SK Gupta, SK Sharma, Z. anorg. allg. Chem. 1980, 471, 203-207.
- ↑ J. Barr, RJ Gillespie, RC Thompson, Inorg. Chem. 1964, 3, 1149.
- ↑ EA Robinson, JA Ciruna: . The chlorosulfuric acid solvent system Part I. Electrical conductivity, transport number, and density measurements on solutions of simple bases . In: Canadian Journal of Chemistry . 46 (10), 1968, p. 1719, doi : 10.1139 / v68-286 .
- ^ RA Robinson, RH Stokes, Electrolyte Solutions, Indian Edn., Academic Press, New York 1959.
- ↑ a b International Nonproprietary Names (INN) for pharmaceutical substances - Names for radicals, groups & others , WHO 2012.
- ↑ BASF SE, EVD 0106 e 2007, The ideal acid for chemical synthesis.
- ↑ L.-S. Tan, KR Srinivasan, SJ Bai, RJ Spry, Journal of Polymer Science A: Polymer Chemistry 1998, 26, 713-724.
- ↑ H. Jie, W. Meixiang, Journal of Polymer Science Part A Polymer Chemistry 1999, 37, 1277-1284.
- ↑ MG Zolotukhin, HM Colquhoun, LG Sestiaa, DR Rueda, D. Flot, Macromolecules 2003, 36, 4766 to 4771.
- ↑ S. Gazeau-Bureau, D. Delcroix, B. Martín-Vaca, D. Bourissou, C. Navarro, S. Magnet, Macromolecules 2008, 41, 3782-3784.
- ↑ K. Schwetlick, Organikum , 23rd edition, Wiley-VCH, Weinheim 2009.
- ↑ a b BASF SE, EVD 0113 d 2005, The "green" acid for cleaning agents.
- ↑ CH Wei, Acta Cryst. 1986, C42, 1839-1842.
- ^ M. O'Meara, A. Alemany, M. Maase, U. Vagt, I. Malkowsky, Metal Finishing 2009: Deposition of Aluminum Using Ionic Liquids , accessed September 17, 2010.
- ↑ LB Zinner, An. Assoc. Bras. Quim. 1979, XXX, 27-32.
- ↑ MS Wickleder, Z. Anorg. General Chem. 2001, 627, 1675-1681.
- ↑ M. de FV de Moura, J. do R. Matos, RF de Farias, Thermochimica Acta 2004, 414, 159-166.
- ↑ M. de FV de Moura, J. do R. Matos, RF de Farias, J. Serb. Chem. Soc. 2006, 71, 905-915.
- ↑ EM Arico, LB Zinner, C. Apostolidis, E. Dornberger, B. Kanellakopulos, J. Rebizant, Journal of Alloys and Compounds 1997, 249, 111-115.
- ↑ EM Arico, LB Zinner, C. Apostolidis, E. Dornberger, B. Kanellakopulos, J. Rebizant, Journal of Alloys and Compounds 2001, 323-324, 39-44.
- ↑ LB Zinner, An. Acad. brasil. Ciênc 1980, 52, 715-722.
- ↑ NC Johnson, JT Turk, WE Bull, HG Mayfield, Jr., Inorganica Chimica Acta 1977, 25, 235-239.
- ↑ I. Rozas, DF Weaver, J. Chem. Soc., Perkin Trans. 2 1996, 461-465.
- ^ RC Paul, VP Kapila, N. Palta, SK Sharma, Indian Journal of Chemistry 1974, 12, 825-826.
- ^ RC Paul, VP Kapila, SK Sharma, J. inorg. nucl. Chem. 1974, 36, 1933-1936.
- ↑ Patent CN101314563 : A method for producing oleic pentaerythritol ester. Filed June 6, 2008 , published December 3, 2008 , applicant: Xiaohan Du, inventor: Xiaohan Du, Songhe Huang.
- ↑ Simon C. Baker, Don P. Kelly, J. Colin Murrell: Microbial degradation of methanesulphonic acid: a missing link in the biogeochemical sulfur cycle . In: Nature . tape 350 , no. 6319 , April 18, 1991, pp. 627-628 , doi : 10.1038 / 350627a0 ( PDF ).


