Dimethyl disulfide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
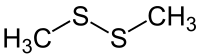
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimethyl disulfide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 6 S 2 | |||||||||||||||
| Brief description |
Highly flammable, volatile, colorless to yellowish liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 94.20 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.06 g cm −3 |
|||||||||||||||
| Melting point |
−85 ° C |
|||||||||||||||
| boiling point |
110 ° C |
|||||||||||||||
| Vapor pressure |
28 mbar (20 ° C) |
|||||||||||||||
| solubility |
heavy in water (2.5 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.5289 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−62.6 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Dimethyl disulfide is a chemical compound from the group of organic disulfides .
Occurrence
Dimethyl disulfide occurs naturally in the form of the odor substance of stinkhorn - which is perceived as very unpleasant - and as one of the causes of the light taste of milk (as a breakdown product of methional ). It is used as a flavoring in a wide variety of foods, such as: B. cabbage and leek species, wine, cheese and beer.
Extraction and presentation
Dimethyl disulfide can be obtained by reacting methanethiol with elemental sulfur .
Together with the monosulfide and the trisulfide, dimethyl disulfide z. B. thermally from sulfur-containing amino acids such as methionine .
properties
Dimethyl disulfide is a flammable, colorless to yellowish liquid with an unpleasant odor. The odor threshold is 7–12 ppb .
The vapors of dimethyl disulfide can form an explosive mixture with air ( flash point 15 ° C, ignition temperature 370 ° C).
use
Dimethyl disulphide is used in oil refineries as a sulphidation agent and in the petrochemical industry as an additive in the cracking of petroleum.
In the USA it is increasingly replacing methyl bromide as a fumigant .
Dimethyl disulfide can also serve as an indicator of microbial contamination. In addition to dimethyl sulfide, the compound is an indicator of mold growth and bacterial growth in sewage pipes.
Analytics
The quantitative determination of dimethyl sulfide is carried out after suitable sample preparation by gas chromatographic headspace analysis with a sulfur-sensitive detector ( Sulfur Chemiluminescence Detector , SCD).
Risk assessment
In 2014, dimethyl disulfide was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The uptake of dimethyl disulfide was caused by concerns about environmental exposure and high (aggregated) tonnage. The reassessment took place from 2014 and was carried out by Germany . A final report was then published.
Related links
Web links
- Entry on Dimethyl disulfide in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
Individual evidence
- ↑ a b c d e f g h i Entry on dimethyl disulfide in the GESTIS material database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-198.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ Odor and color of the rod mushrooms .
- ^ Alfred Töpel: Chemistry and Physics of Milk. Behr's Verlag DE, 2015, ISBN 978-3-954-68360-4 , p. 706 ( limited preview in Google book search).
- ↑ a b c d Entry on dimethyl disulfide. In: Römpp Online . Georg Thieme Verlag, accessed on February 28, 2017.
- ↑ EPA Pesticide Fact Sheet Dimethyl Disulfide, July 9, 2010, p. 7, accessed October 13, 2014.
- ↑ Arkema: Dimethyl Disulfide , accessed February 28, 2017.
- ↑ DMDS for agricultural soil fumigation
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance occurrence, properties and use of fragrances and their mixtures . Springer-Verlag, 2015, ISBN 978-3-658-07310-7 , pp. 83 ( limited preview in Google Book search).
- ^ Karl Höll: Water use in the cycle: hygiene, analysis and evaluation . Walter de Gruyter, 2011, ISBN 978-3-11-022678-2 , p. 509 ( limited preview in Google Book search).
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Dimethyl disulphide , accessed on March 26, 2019.




