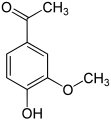
Apocynin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
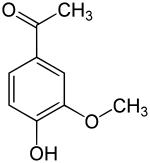
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Acetovanillon | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 9 H 10 O 3 | |||||||||||||||||||||
| Brief description |
white needles |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 166.18 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
112-115 ° C |
|||||||||||||||||||||
| boiling point | ||||||||||||||||||||||
| solubility |
soluble in hot water as well as ethanol, ether, chloroform and benzene |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Apocynin (usually also acetovanillon , 4-hydroxy-3-methoxyacetophenone ) is an organic-chemical compound with the empirical formula C 9 H 10 O 3 , which is structurally derived from both acetophenone and guaiacol ( o-methoxyphenol ). It is a derivative of acetophenone with an additional hydroxyl and methoxy group as substituents . Structurally, it is also one of the relatives of vanillin . It has been isolated from a variety of herbal sources and is being studied for its numerous pharmacological properties.
In the biological-pharmaceutical environment, the term apocynin is used, in chemistry, due to its structural relationship with vanillin, acetovanillon is more used .
history

Apocynin was first described by the German pharmacologist Oswald Schmiedeberg in 1883 and isolated for the first time from the roots of Indian hemp or Canadian hemp ( Apocynum cannabinum ). At that time, this plant was already known for its effectiveness against edema and heart problems.
In 1891 Ferdinand Tiemann published his work on the isolation of acetovanillon. It was through him that the name was chosen: … Acetovanillon has exactly the same relationship to vanillin as acetophenone has to benzaldehyde. Taking this analogy into account, I have named the compound acetovanillon and named its derivatives accordingly. In the work immediately following Erich Neitzel, this analogy of name choice is used for the derivatives, e.g. E.g .: acetoveratrone ( 3,4-dimethoxyacetophenone , derived from veratrum aldehyde ) and acetoprotocatechone ( 3,4-dihydroxyacetophenone , derived from protocatechu aldehyde ). Th. Otto in turn describes the synthesis by reacting guaiacol with glacial acetic acid in the presence of zinc chloride and aluminum chloride in the following article :
Finnemore turned back to isolation in 1908, improving the process and yield. He also showed that apocynin and the acetovanillon synthesized by Otto in 1891 are the same substance.
In 1971, apocynin was also isolated from Picrorhiza kurroa , a small plant that grows at high altitudes in the western Himalayas. P. kurroa has been used at times to treat liver and heart problems, jaundice, and asthma. Apocynin has anti-inflammatory properties and prevents the formation of free radicals , oxygen ions and peroxides in the body. Apocynin has since been extensively studied for its properties and utility in the fight against disease.
properties
Acetovanillon forms white needles and is soluble in hot water, as well as ethanol, ether, chloroform, and benzene. It melts at 112–115 ° C and boils at 295–300 ° C at normal pressure or 263–265 ° C at negative pressure (17 mmHg ; ≈ 23 hPa ). An aqueous solution of iron (III) chloride and acetovanillon form an intense blue-violet color.
As a structural cousin of vanillin, it is part of vanilla flavors. The vanilla flavor obtained on the basis of lignin has a richer taste profile. This is due to the presence of acetovanillon ( apocynin ) as a lignin by-product - an impurity that does not occur in vanillin from a guaiacol synthesis.
Isomers
iso- acetovanillon ( 3-hydroxy-4-methoxyacetophenone ) differs from acetovanillon in the position of the methoxy group. Instead of position 3, this is found here in position 4. Hydroxy and methoxy groups swap places compared to acetovanillon. The structural analogy corresponds to that between vanillin and isovanillin .
ortho- acetovanillon ( 2-hydroxy-3-methoxyacetophenone ) differs from acetovanillon in the position of the hydroxyl group. The prefix ortho indicates the position of the hydroxyl group in relation to the acetyl group ; in acetovanillon these two groups are in the para position. The structural analogy corresponds to that between vanillin and ortho- vanillin .
pharmacology
Apocynin is an inhibitor ( inhibitor ) of the NADPH oxidase (NOX). This membrane-bound enzyme can be found u. a. in phagosomes and reduces oxygen (O 2 ) to superoxide (O 2 - · ) with consumption of NAD (P) H . Hyperoxide belongs to the reactive oxygen species (ROS) and is formed by leukocytes and granulocytes in the course of the immune system to kill bacteria and fungi . Apocynin selectively inhibits NADPH oxidase without affecting other immunospecific tasks.
In addition, the specificity of apocynin as a selective inhibitor of NADPH oxidase was demonstrated in a study on renal medulla cells. The NADPH oxidase is activated in these tissue cells by an outflow of protons. Apocynin, however, specifically inhibits this enzyme without affecting the flow of protons.
- Case studies of a possible therapeutic effect
- Neutrophil granulocytes play a crucial role in the disease of collagen-induced arthritis . They are also involved in developing inflammatory reactions in the joints. Apocynin is said to reduce the number of these granulocytes. However, an existing inflammation cannot be reversed.
- In clinical studies, apocynin has shown a reduction in damage to the intestine in rats with inflammatory bowel disease . It also lowers the enzymatic activity of myeloperoxidase , which is involved in the inflammatory reaction in a disease. Finally, apocynin also lowers the amount of macrophages and granulocytes in the gut.
- Androsene , the glucoside of apocynin, appears to have an effect in the treatment of asthma diseases . This prevents constriction of the bronchi in guinea pigs . Apocynin may inhibit the development of certain inflammatory processes.
- In the treatment of atherosclerosis , apocynin could be used to inhibit the activity of NADPH oxidase. As a result, fewer ROS are generated, which damage endothelial cells.
- In patients with amyotrophic lateral sclerosis (ALS), a degenerative disease of the motor nervous system , various mutations in the superoxide dismutase (SOD-1) gene have been found, which disproportionate hyperoxide to hydrogen peroxide (H 2 O 2 ) and O 2 . The lifespan of mice suffering from ALS with such a defective SOD-1 gene is increased by apocynin given to drinking water. In addition, the toxicity of glial cells in cell cultures with a defective SOD-1 gene is specifically reduced by apocynin. This toxicity is caused by the fact that hyperoxides are produced by NADPH oxidases (Nox) in glial cells.
It is possible that SOD-1 serves as a self-regulating redox sensor against hyperoxide, which is generated by the NADPH oxidase. Although apocynin causes septic granulomatosis in humans, new drugs for the therapy of ALS could be developed on the basis of these results - initially in mice.
Individual evidence
- ↑ a b c d Ferd. Tiemann: "About Acetovanillon"; in: Reports of the German Chemical Society ; 1891 ; 24 (2); Pp. 2855-2862; doi: 10.1002 / cber.189102402110 .
- ↑ a b c d e f data sheet 4′-Hydroxy-3′-methoxyacetophenone from Sigma-Aldrich , accessed on November 9, 2012 ( PDF ).
- ↑ a b c d Erich Neitzel: “About derivatives of acetovanillons”; in: Reports of the German Chemical Society ; 1891 ; 24 (2); Pp. 2863-2868; doi: 10.1002 / cber.189102402111 .
- ↑ a b Entry on apocynin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Otto Schmiedeberg: "About the effective components of the root of Apocynum canabinum L."; in: Arch. Exp. Path. Pharm .; 1883 ; 16 ; Pp. 161-164.
- ↑ HC Wood: "A study of Apocynum cannabinum"; in: J. Am. Med. Assoc. ; 1904 ; 43 ; Pp. 1953-1957; Abstract ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ↑ Erich Neitzel: "The Acetovanillon and Its Derivatives" (Diss.); Berlin 1890; Pressure v. Thormann & Goetsch; 40 pp. + 2 pp.
- ↑ Th. Otto: "On the synthesis of acetovanillons from guaiacol and glacial acetic acid"; in: Reports of the German Chemical Society ; 1891 ; 24 (2); Pp. 2869-2870; doi: 10.1002 / cber.189102402112 .
- ↑ H. Finnemore: “The constituents of Canadian hemp; Part I: Apocynin “; in: J. Chem. Soc. ; 1908 ; 93 ; Pp. 1513-1520; doi: 10.1039 / CT9089301513 .
- ↑ K. Basu, B. Das Gupta, SK Bhattacharya, PK Debnath: "Chemistry and pharmacology of apocynin, isolated from Picrorhiza kurroa Royle ex Benth"; in: Current Science ; 1971 ; 40 (22); Pp. 603-604.
- ^ Jos M. Simons, Bert A. 't Hart, Theo. RAM Ip Vai Ching, Hans van Dijk, Rudi P. Labadie: "Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils"; in: Free Radical Biology and Medicine ; 1990 ; 8 (3); Pp. 251-258; doi: 10.1016 / 0891-5849 (90) 90070-Y ; PMID 2160411 .
- ↑ Lawrence J. Esposito, K. Formanek, G. Kientz, F. Mauger, V. Maureaux, G. Robert, F. Truchet: "Vanillin", Kirk-Othmer Encyclopedia of Chemical Technology , 4th edition, Vol. 24, John Wiley & Sons , New York 1997, pp. 812-825.
- ^ Wilhelm Schneider, Edgar Kraft: "Sulpho-acetic acid as a condensing agent, IV .: Iso-acetovanillon"; in: Reports of the German Chemical Society ; 1922 ; 55 (6); Pp. 1892-1899; doi: 10.1002 / cber.19220550640 .
- ^ Tadeus Reichstein: "Acetovanillon, iso- and ortho-Acetovanillon. (A case of acyl migration to meta position) ”; in: Helvetica Chimica Acta ; 1927 ; 10 (1); Pp. 392-397; doi: 10.1002 / hlca.19270100147 .
- ^ N. Li, G. Zhang, FX Yi, AP Zou, PL Li: "Activation of NAD (P) H oxidase by outward movements of H + ions in renal medullary thick ascending limb of Henle"; in: Am J Physiol Renal Physiol. ; 2005 ; 289 (5); F1048-F1056; PMID 15972387 .
- ↑ Bert A. 't Hart, Jos M. Simons, Shoshan Knaan-Shanzer, Nicolaas PM Bakker, Rudi P. Labadie: "Anti-arthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin"; in: Free Radical Biology and Medicine ; 1990 ; 9 (2); Pp. 127-131; doi: 10.1016 / 0891-5849 (90) 90115-Y ; PMID 2172098 .
- ↑ MJHJ Palmen, CJ Beukelman, RGM Mooij, AS Peña, EP by Rees: “Anti-inflammatory effect of apocynin, a plant-derived NADPH oxidase antagonist, in acute experimental colitis”; in: The Netherlands Journal of Medicine ; 1995 ; 47 (2); A41; doi: 10.1016 / 0300-2977 (95) 97051-P .
- ^ Edwin van den Worm, Cees J. Beukelman, Albert JJ van den Berg, Burt H. Kroes, Rudi P. Labadie, Hans van Dijk: "Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils "; in: Eur. J. Pharmacol. ; 2001 ; 433 (2-3); Pp. 225-230; doi: 10.1016 / S0014-2999 (01) 01516-3 ; PMID 11755156 .
- ^ Elisabeth A. Peters, Jeroen TN Hiltermann, Jan Stolk: "Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics"; in: Free Radical Biology and Medicine ; 2001 ; 31 (11); Pp. 1442-1447; doi: 10.1016 / S0891-5849 (01) 00725-0 ; PMID 11728816 .
- ↑ Maged M. Harraz, Jennifer J. Marden, Weihong Zhou, Yulong Zhang, Aislinn Williams, Victor S. Sharov, Kathryn Nelson, Meihui Luo, Henry Paulson, Christian Schöneich, John F. Engelhardt: “SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model "; in: J. Clin. Invest. ; 2008 ; 118 (2); Pp. 659-670; PMID 18219391 ; PMC 2213375 (free full text, PDF).
literature
- Cees J. Beukelman, Edwin van den Worm, C. Henriette, Q. van Ufford, Burt H. Kroes, Albert JJ van den Berg: “Discovery of new anti-inflammatory drugs from plant origin”; in: Annals of Gastroenterology ; 2002 ; 15 (4); Pp. 320-323; Abstract ; PDF .
- J. Stefanska, R. Pawliczak: “Apocynin: Molecular Aptitudes”; in: Mediators of Inflammation ; 2008 ; Article ID 106507, 10 pages; doi: 10.1155 / 2008/106507 .
- E. Worm: Apocynin: A tiny, but mighty molecule , 2001 (PDF; 547 kB).