Acetosyringone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
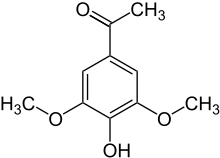
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Acetosyringone | ||||||||||||||||||
| other names |
3,5-dimethoxy-4-hydroxyacetophenone |
||||||||||||||||||
| Molecular formula | C 10 H 12 O 4 | ||||||||||||||||||
| Brief description |
colorless prisms |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 196.20 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
123-124 ° C |
||||||||||||||||||
| pK s value |
7.8 ± 0.2 |
||||||||||||||||||
| solubility |
bad in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Acetosyringone ( 3,5-dimethoxy-4-hydroxyacetophenone ) is a phenolic natural substance and an organic-chemical compound with the empirical formula C 10 H 12 O 4 , which is structurally derived from both acetophenone and syringol ( 2,6-dimethoxyphenol ) . It is a derivative of acetophenone with an additional hydroxyl and two methoxy groups as substituents . The name of the compound comes - analogous to syringol, syringa alcohol , syringaaldehyde or syringic acid - from the Latin name of lilac ( syringa ).
properties
The powder, which is light brown when not pure, does not dissolve very much in water. Acetosyringone is one of the substances secreted by wounded plant cells and is therefore a secondary plant substance . As a pheromone , it also plays a role in the metabolism of insects. Agrobacterium tumefaciens is attracted by acetosyringone through binding to the receptor and virulence factor virA , whereby the gene expression of the virulence factors is increased and the plant can be infected via the injury. Acetosyringone increases the formation of mycorrhiza from glomus intraradices . The total synthesis was described in 1956 by LW Crawford.
use
Acetosyringone is important for plant biotechnology. It is used there as an additive (usually 200 µM ) in the transformation with agrobacteria , because like several other phenol derivatives it is able to cause an infection with the bacterium Agrobacterium tumefaciens , which can be used to smuggle any genetic material into the plant . This applies in particular to dicotyledonous plants ( dicots ).
In addition, acetosyringone can increase the activity of peroxidases and oxidases .
See also
Individual evidence
- ↑ a b Entry on acetosyringone. In: Römpp Online . Georg Thieme Verlag, accessed on April 15, 2014.
- ↑ Feng Xu: Effects of Redox Potential and Hydroxide Inhibition on the pH Activity Profile of Fungal Laccases , Journal of Biological Chemistry , Vol. 272, No. 2, pp. 924-928 ( PDF ).
- ↑ a b c data sheet Acetosyringone (PDF) from Carl Roth , accessed on March 14, 2017.
- ↑ Acetosyringone on pherobase .
- ↑ JR Aldrich, MS Blum, SS Duffey, HM Fales: Male specific natural products in the bug, Leptoglossus phyllopus: Chemistry and possible function . In: Journal of Insect Physiology . 22, No. 9, 1976, pp. 1201-1206. doi : 10.1016 / 0022-1910 (76) 90094-9 .
- ↑ JR Aldrich, MS Blum, HM Fales: Species-specific natural products of adult male leaf-footed bugs (Hemiptera: Heteroptera) . In: Journal of Chemical Ecology . 5, 1979, p. 53. doi : 10.1007 / BF00987687 .
- ↑ Baker C. Jacyn, Mock Norton M., Whitaker Bruce D., Roberts Daniel P., Rice Clifford P., Deahl Kenneth L. and Aver'Yanov Andrey A .: Involvement of acetosyringone in plant-pathogen recognition . In: Biochemical and biophysical research communications . 328, No. 1, 2005, pp. 130-136. doi : 10.1016 / j.bbrc.2004.12.153 . PMID 15670760 .
- ↑ B. Schrammeijer, A Beijersbergen, KB Idler, LS Melchers, DV Thompson, PJ Hooykaas: Sequence analysis of the vir-region from Agrobacterium tumefaciens octopine Ti plasmid pTi15955 . In: Journal of Experimental Botany . 51, No. 347, 2000, pp. 1167-1169. doi : 10.1093 / jexbot / 51.347.1167 . PMID 10948245 .
- ↑ Estela Flores-Gómez, Lidia Gómez-Silva, Roberto Ruiz-Medrano, Beatriz Xoconostle-Cázares: Role of acetosyringone in the accumulation of a set of RNAs in the arbuscular mycorrhiza fungus Glomus intraradices . In: International Microbiology . 11, No. 4, 2008, pp. 275-282. doi : 10.2436 / 20.1501.01.72 . PMID 19204900 .
- ↑ LW Crawford, EO Eaton, JM Pepper: An Improved Synthesis of Acetosyringone . In: Canadian Journal of Chemistry . 34, No. 11, 1956, pp. 1562-1566. doi : 10.1139 / v56-204 .
- ↑ Shahla N. Sheikholeslam, Donald P. Weeks: Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens . In: Plant Molecular Biology . 8, No. 4, 1987, p. 291. doi : 10.1007 / BF00021308 .
- ↑ Agro Infiltration
- ↑ Agrobacterium-mediated Transformation ( Memento of the original from March 29, 2007 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ US Patent 6483013 .
- ↑
- ^ Ali Movahedi, Jiaxin Zhang, Rasoul Amirian, Qiang Zhuge: An Efficient Agrobacterium-Mediated Transformation System for Poplar. In: International Journal of Molecular Sciences. 15, 2014, p. 10780, doi : 10.3390 / ijms150610780 .
- ↑ US Patent 5912405 .
Web links
- Entry on acetosyringone . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 17, 2012.
