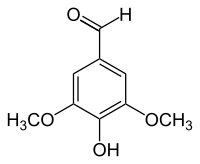
Syringaldehyde
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Syringaldehyde | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 10 O 4 | ||||||||||||||||||
| Brief description |
Pale yellow needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 182.17 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
113 ° C |
||||||||||||||||||
| boiling point |
192–193 ° C (19 hPa ) |
||||||||||||||||||
| pK s value |
7.0 ± 0.2 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Syringaldehyde , rarely syringaldehyde ( 3,5-dimethoxy-4-hydroxybenzaldehyde , FEMA 4049 ) is an organic chemical compound with the empirical formula C 9 H 10 O 4 . It is a derivative of benzaldehyde with one additional hydroxy and two methoxy groups as substituents . The name of the aldehyde comes from the Latin name for lilac ( Syringa ).
History and occurrence
The aldehyde was first in 1889 in the lilac Syringa vulgaris occurring glucoside syringin by oxidation and hydrolysis recovered. Syringaldehyde is also found in pineapple, beer, brandy, rum, many different types of whiskey, sherry, roasted barley and hardwood smoke.
Structural formula of syringin
(β- D -glucoside of sinapyl alcohol )
Extraction and presentation
Natural sources
Syringaldehyde is formed - often in addition to considerable amounts of vanillin - during the oxidative degradation of lignin , albeit with strongly fluctuating yields, which depend crucially on the biomass used and the reaction conditions.
Chemical syntheses
Syringaldehyde can be produced chemically by the reaction of vanillin with iodine to form 5-iodovanillin and nucleophilic substitution of the iodine by a methoxy group in the presence of a copper catalyst. Much more economical is the route via 5-bromvanillin , which can be obtained from vanillin either by complete bromination in the presence of an imide in a two-phase aqueous organic solvent mixture and reoxidation of HBr with chlorine or homogeneously in 95% yield in methanol . However, bromine- methanol mixtures are themselves reactive.
The bromine atom is exchanged for a methoxy group by electron transfer catalysis by copper salts under comparatively mild reaction conditions (3 hours at 125 ° C) in methanol / sodium methoxide in an autoclave under catalysis with a system of basic copper carbonate and carbon dioxide in 99% yield. or in dimethylformamide with sodium methoxide with copper (I) chloride in a total yield of 86% based on vanillin. A simpler, pressureless reaction of the transition metal -induced nucleophilic substitution only allows the use of copper (I) complexes in DMF.
Alternatively, syringaldehyde can be obtained by regioselective demethylation of 3,4,5-trimethoxybenzaldehyde in acid with sulfuric acid in 96% yield, with aluminum chloride in 95% yield and in the basic with aqueous dimethylamine under pressure in 72% yield.
Synthesis by the Duff reaction is also possible .
Starting from the industrially available p -cresol , syringaldehyde is produced in a three-stage synthesis via 2,6-dibromo-4-methylphenol (96.5% yield), 2,6-dimethoxy-4-methylphenol (84%) and oxidation of the methyl group ( 91%) in an overall yield of 63 to 67%.
properties
Physical Properties
Syringaldehyde is a solid that crystallizes in pale yellow needles, which is almost insoluble in water but dissolves in ethanol , diethyl ether and glacial acetic acid . It melts at 110–113 ° C and boils at 192–193 ° C under reduced pressure (19 hPa ).
Chemical properties
The substance is structurally derived from both benzaldehyde and syringol ( 2,6-dimethoxyphenol ). Due to its bifunctional character, syringaldehyde is very reactive. A large number of derivatives can be synthesized by etherification , esterification or aldol condensation .
The pK s value of the phenolic OH group of the Syringaldehyds is 7.0 ± 0.2. This value is significantly lower than the Syringol at 9.98. The electron-withdrawing aldehyde group increases the OH acidity through its −M effect ; the phenolic OH bond is increasingly polarized. Similarly, in comparison of 4-hydroxybenzaldehyde with a pK s value of 7.66 to phenol with 9.99, as well as vanillin having a pK s value of 7.40 to guaiacol ( 2-methoxyphenol ) 9, 98
Structural and name relatives
 |
 |
 |
 |

|
| Syringol | Syringa alcohol | Syringaldehyde | Syringic acid | Acetosyringone |
use
The aldehyde is used as an intermediate in chemical syntheses and as a fragrance in perfumery.
Syringaldehyde is attracting increasing interest because of its diverse bioactivities as well as because of its usefulness as a molecular building block for various active ingredient syntheses. As a natural antioxidant , syringaldehyde effectively inhibits the oxidation of unsaturated oils and lecithins.
Syringaldehyde also inhibits the growth of the xylitol- producing yeast Candida guilliermondii. The previously determined antimicrobial and enzyme-inhibiting activities of syringaldehyde are relatively weak compared to standard reference substances. Since syringaldehyde is also used in the pyrolysis of lignin, e.g. For example, when hardwood is burned , syringaldehyde can be used as a molecular marker for smoke emissions into the atmosphere.
Syringaldehyde is a starting material for 3,4,5-trimethoxybenzaldehyde , a key compound in the synthesis of the antibiotic trimethoprim . According to a more recent procedure, the target compound is obtained from p -cresol via the sodium salt of syringaldehyde in an overall yield of 67.4%.
The Knoevenagel reaction of syringaldehyde with bis (2-ethylhexyl) malonate (by transesterification of diethyl malonate with 2-ethylhexanol ) in the presence of piperidine - acetic acid gives bis (2-ethylhexyl) -3,5-dimethoxy-4 in 91% yield -hydroxy-benzylidene malonate (DESM, Oxynex ST).
It is used as a photo stabilizer and antioxidant in cosmetic preparations, especially for stabilizing UV filters, such as. B. Avobenzone in sunscreens .
The catalytic hydrogenation of the benzylidene function leads to bis (2-ethylhexyl) -3,5-dimethoxy-4-hydroxy-benzylmalonate (HDBM, Ronacare AP), which is triggered by UV, VIS and near-infrared light, causing the formation of reactive oxygen radicals (ROS ) is effectively suppressed and is therefore used in cosmetics with a light protection function.
Web links
Individual evidence
- ↑ a b Entry on FEMA 4049 in the database of the Flavor and Extract Manufacturers Association of the United States .
- ↑ a b c d e f g h i j k Entry on syringaldehyde. In: Römpp Online . Georg Thieme Verlag, accessed on March 14, 2017.
- ↑ a b Feng Xu: Effects of Redox Potential and Hydroxide Inhibition on the pH Activity Profile of Fungal Laccases , Journal of Biological Chemistry , Vol. 272, No. 2, pp. 924-928 ( PDF ).
- ↑ a b Data sheet 3,5-dimethoxy-4-hydroxybenzaldehyde (PDF) from Merck , accessed on January 29, 2012.
- ↑ a b c d e data sheet Syringaldehyde, ≥ 98% from Sigma-Aldrich , accessed on March 14, 2017 ( PDF ).
- ^ R. Sun, J. Tomkinson, FC Mao, XF Sun: Physicochemical characterization of lignins from rice straw by hydrogen peroxide treatment . In: J. Appl. Polym. Sci. tape 79 , no. 4 , 2001, p. 719-732 , doi : 10.1002 / 1097-4628 (20010124) 79: 4 <719 :: AID-APP170> 3.0.CO; 2-3 .
- ↑ J. Zhang, H. Deng, L. Lin: Wet Aerobic Oxidation of Lignin into Aromatic Aldehydes Catalysed by a Perovskite-type Oxide: LaFe 1-x Cu x O 3 (x = 0, 0.1, 0.2) . In: Molecules . tape 14 , 2009, p. 2747-2757 , doi : 10.3390 / molecules14082747 .
- ↑ PC Rodrigues Pinto, EA Borges da Silva, AE Rodrigues: Insights into Oxidative Conversion of Lignin to High-Added-Value Phenolic Aldehydes . In: Ind. Eng. Chem. Res. Volume 50 , no. 2 , 2010, p. 741-748 , doi : 10.1021 / ie102132 .
- ↑ JM Pepper, JA MacDonald: The synthesis of syringaldehyde from vanillin . In: Can. J. Chem. Volume 31 , no. 5 , 1953, pp. 476-483 , doi : 10.1139 / v53-064 .
- ↑ a b c EP 155 335 (1985) Ahrens K.-H. Liebenow W., Count I. (Ludwig Heuman & Co GmbH); Process for the preparation of 3,5-dimethoxy-4-alkoxybenzaldehydes .
- ^ A b Percy S. Manchand, Peter S. Belica, Harry S. Wong: Synthesis of 3,4,5-Trimethoxybenzaldehyde , in: Synth. Commun. , 1990, 20 (17), pp. 2659-2666 ( doi: 10.1080 / 00397919008051474 ).
- ^ Paul T. Bowman, Edmond I. Ko, and Paul J. Sides: A Potential Hazard in Preparing Bromine-Methanol Solutions , in: Journal of the Electrochemical Society , 1990, 137 , pp. 1309-1311 Archived copy ( Memento from 22 April 2017 in the Internet Archive ).
- ↑ D. Nobel: The copper-carbon dioxide system, a new mild and selective catalyst for the methoxylation of non-activated aromatic bromides . In: J. Chem. Soc., Chem. Commun. 1993, p. 419-420 , doi : 10.1039 / C39930000419 .
- ^ IA Pearl, DL Beyer: Reactions of Vanillin and its Derived Compounds. XVII. A Synthesis of Syringaldehyde from Vanillin . In: J. Am. Chem. Soc. tape 74 , no. 17 , 1952, pp. 4262-4263 , doi : 10.1021 / ja01137a006 .
- ↑ S. Madhavi, Design, synthesis, biochemical and biological evaluation of benzocyclic and enediyne analogs of combretastatins as potential tubulin binding ligands in the treatment of cancer, Ph.D. Thesis, Baylor University, 2007, p. 102 ( abstract ( memento of March 4, 2016 in the Internet Archive )).
- ↑ Patent US20110245544A1 : Process for preparing hydroxy-substituted aromatic aldehydes. Registered on April 1, 2011 , published on October 6, 2011 , applicant: BASF SE, inventor: K. Ebel, S. Rüdenauer.
- ↑ CFH Allen, Gerhard W. Leubner: Syringic Aldehyde In: Organic Syntheses . 31, 1951, p. 92, doi : 10.15227 / orgsyn.031.0092 ; Coll. Vol. 4, 1963, p. 866 ( PDF ).
- ↑ AK Tripathi, JK Sama, SC Taneja: An expeditious synthesis of syringaldehyde from p-cresol . In: Indian J. Chem. 49B, 2010, p. 379-381 ( PDF ).
- ↑ Ragnar, M .; Lindgren, CT, Nilvebrant N.-O .: pK a -values of Guaiacyl and Syringyl Phenols Related to Lignin , J. Wood Chem. Technol. 2000, 20 (3), pp. 277-305 ( doi: 10.1080 / 02773810009349637 , excerpt ).
- ↑ a b c d CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b M.NM Ibrahim, RB Sriprasanthi, S. Shamsudeen, F. Adam, SA Bhawani: A concise review of the natural existence, synthesis, properties, and applications of syringaldehyde . In: Bioresources . tape 7 , no. 3 , 2012, p. 4377-4399 ( abstract ).
- ↑ OG Boundagiou, SA Ordoudi, MZ Tsimidou: Structure-antioxidant activity relationship study of natural hydroxybenzaldehydes using in vitro assays . In: Food Res. Int. tape 43 , no. 8 , 2010, p. 2014–2019 , doi : 10.1016 / j.foodres.2010.05.021 .
- ↑ C. Kelly, O. Jones, C. Barnhart, C. Lajoie: Effect of furfural, vanillin and syringaldehyde on Candida guilliermondii growth and xylitol biosynthesis . In: Appl. Biochem. Biotechnol. tape 148 , no. 1–3 , 2008, pp. 97-108 , doi : 10.1007 / s12010-007-8103-1 .
- ↑ AL Robinson, R. Subramanian, NM Donahue, A. Bernardo-Bricker, WF Rogge: Source Apportionment of Molecular Markers and Organic Aerosol. 2. Biomass smoke . In: Environ. Sci. Technol. tape 40 , no. 24 , 2006, pp. 7811-7819 , doi : 10.1021 / es060782h .
- ^ MA Bari, G. Baumbach, B. Kuch, G. Scheffknecht: Air Pollution in Residential Areas from Wood-fired Heating . In: Aerosol Air Qual. Res. Volume 11 , 2011, p. 749-757 , doi : 10.4209 / aaqr.2010.09.0079 .
- ↑ Y.-F. Ji, Z.-M. Zong, X.-Y. Wei: Efficient and convenient synthesis of 3,4,5-trimethoxybenzaldehyde from p-cresol . In: Synth. Commun. tape 32 , no. 18 , 2002, p. 2809-2814 , doi : 10.1081 / SCC-120006464 ( erowid.org ).
- ↑ Patent US6602515B1 : Photo stable organic sunscreen compounds with antioxidant properties and compositions obtained therefrom. Filed July 16, 2001 , published August 5, 2003 , applicant: EM Industries, inventor: RT Chaudhuri.
- Jump up ↑ RK Chaudhuri, Z. Lascu, G. Puccetti, AA Deshpande, SK Paknikar: Design of a photostabilizer having built-in antioxidant functionality and its utility in obtaining broad-spectrum sunscreen formulations . In: Photochem. Photobiol. tape 82 , no. 3 , 2006, p. 823-828 , doi : 10.1562 / 2005-07-15-RA-612 .
- ↑ Patent US8106233B2 : Antioxidant Compounds. Registered on March 21, 2006 , published on January 31, 2012 , applicant: Merck Patent GmbH, inventor: T. Rudolph, H. Buchholz.
- ↑ T. Rudolph, S. Eisenberg, J. Grumelard, B. Herzog: State-of-the-art Light Protection against Reactive Oxygen Species . In: SOFW-Journal . tape 140 , no. 3 , 2014, p. 9-14 ( shopsofw.com ).







