Oseltamivir
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
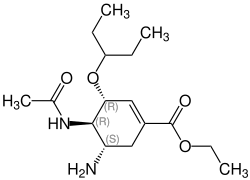
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Oseltamivir | ||||||||||||
| other names |
(3 R , 4 R , 5 S ) -4-Acetamido-5-amino-3- (1-ethylpropoxy) cyclohex-1-en-1-carboxylic acid ethyl ester |
||||||||||||
| Molecular formula | C 16 H 28 N 2 O 4 | ||||||||||||
| Brief description |
odorless solid (phosphate) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 312.40 g · mol -1 (oseltamivir) | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
197-204 ° C (phosphate) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Oseltamivir is a drug from the group of neuraminidase inhibitors , which for the treatment of influenza ( influenza ) and for post-exposure prophylaxis is permitted (prevention of possible contact with an infected person) in children aged one year and adults. In addition to zanamivir and amantadine, oseltamivir is offered as a remedy for the real flu caused by influenza A or B viruses . It is supposed to have a virostatic effect, that is, to prevent viruses from multiplying in the body; however, it cannot eliminate or inactivate them . The use against the avian flu H5N1 was recommended by the WHO. In 2012 and 2014, research by the Cochrane Collaboration revealed doubts about the efficacy and safety of oseltamivir. The WHO has therefore oseltamivir in 2017 of "essential" ( English essential ) to only "supplement" (Engl .: complementary ) downgraded.
history
Influenza drug development began in the early 1990s. Scientists from the Parkville campus for pharmacy at Monash University in Melbourne , Australia , presented an unapproved precursor to zanamivir as an anti-flu agent in mice at an infectious disease congress in Los Angeles on October 14, 1992 . However, this active ingredient had to be inhaled through the lungs, which is where the disease usually breaks out. Norbert Bischofberger from the biotechnology company Gilead Sciences in Foster City near San Francisco tried to develop a drug that works on the same principle, but can be administered in tablet form. After this was successful, a collaboration with the Roche company began. In November 1996, clinical trials for drug approval began .
Oseltamivir was first approved in Switzerland in September 1999. In the European Union, Hoffmann-La Roche's application for drug approval was withdrawn again in 2000 because members of the European Medicines Committee had doubts about the evidence of the benefits. Roche wanted to submit study data later.
In December 2000 oseltamivir was officially approved as an active ingredient against influenza in the USA and Japan, and then in June 2002 in the European Union.
Approval was initially restricted: to prevent flu, it could only be used in patients aged 13 and over. This restriction has now been lifted and oseltamivir can also be given to children over the age of one for prevention . The supplementary application for approval was based on the results of a clinical study on home flu treatment with the active ingredient. The study, in which over 1,000 patients - adults and children - took part, found that oseltamivir prevents other people in the same household from getting the flu. The protective effect in children between 1 and 12 years was just as good as in the other age groups.
In 2006 Roche increased sales of Tamiflu (trade name) year-on-year by 68% to 2.6 billion Swiss francs. Tamiflu was thus in fourth place among Roche's top-selling drugs in 2006 (2005: 6th place). In 2008, sales of Tamiflu fell to 278 million francs per quarter, and in the first half of 2011, Tamiflu had sales of 262 million francs and in the first half of 2018, 320 million francs.
effectiveness
The effectiveness of oseltamivir is low. A meta-analysis by the Cochrane Collaboration at the beginning of 2012 came to the conclusion, among other things, that the substance only shortened the persistence of flu symptoms by 21 hours and had no influence on the frequency with which sick people were admitted to hospital. A meta-analysis by the Cochrane Collaboration from 2014 found that treatment with oseltamivir in adults reduced the duration of illness from 7 to 6.3 days. However, oseltamivir had no influence on the frequency of severe forms such as pneumonia or bronchitis and had adverse drug effects such as nausea, vomiting and, when taken prophylactically, headache, psychiatric and kidney-damaging effects. In particular, the use of oseltamivir failed to reduce the proportion of patients who had to be hospitalized. They also referred to mental and neurological disorders that had appeared in studies but had not been published. From this, the researchers concluded that oseltamivir is less effective and has more severe side effects than the manufacturer claims.
A systematic report published in The Lancet in January 2006 , which is based on two Cochrane Reviews (systematic reviews) published in 1999 and 2004 on the prevention and therapy of influenza, summarized the evidence of antiviral therapy. Essentially, T. Jefferson et al. a. that neuraminidase inhibitors cannot prevent an infection with influenza, but can alleviate the course. This does not apply to the influenza-like illness, i.e. the situation in which there is no virus test and other viruses are possible causes. The preparation oseltamivir also reduces the likelihood of the influenza spreading further in the home environment. This was explained by a reduction in virus excretion through the nose, which is primarily responsible for the transmission of the infection. However, the virus colonization in the nose is not completely eliminated .
The authors therefore assumed that the sole use of neuraminidase inhibitors in a pandemic would not be sufficient to control the spread due to the much higher viral load in such a situation . Rather, an overly optimistic assessment of the effectiveness of neuraminidase inhibitors could lead to increased risk behavior and thus even to a promotion of the spread of the virus. The use of neuraminidase inhibitors during an influenza epidemic is therefore only promising with additional protective measures such as isolation or protective clothing. The routine use of neuraminidase inhibitors in the usual "flu waves" was not recommended because of their lack of effectiveness in flu-like diseases. Of amantadine and rimantadine the unfavorable side effect profile and the development of resistance has been discouraged due. Oseltamivir also appears to be a justifiable drug for pregnant women and nursing mothers. Following a new study, the FDA decided that Tamiflu (oseltamivir) can also be given to infants.
Manufacturing
The starting material for the production of oseltamivir is shikimic acid in the natural (3 R , 4 S , 5 R ) configuration . Today this is mainly obtained from a genetically engineered, special strain of Escherichia coli bacteria, but it can also be extracted from real star anise . 30 kg of anise make about 1 kg of shikimic acid. The E. coli bacteria produce shikimic acid when they are "overfed" with glucose . The extracted raw material is then filtered and dried. In the multi-stage synthesis , u. a. highly explosive azides as intermediates. The production of oseltamivir from the raw material is very complex.
A research group at Tokyo University , including Masakatsu Shibasaki , was able to synthesize oseltamivir in 2006 from a common laboratory chemical, 1,4-cyclohexadiene .
In the following years, further - also stereospecific - synthetic routes were developed.
E. Corey's working group did not claim patent protection for the latter synthesis in order to enable production for everyone.
Mode of action
After oral ingestion, more than 75% of the active ingredient oseltamivir is quickly absorbed into the blood by the organism in the gastrointestinal tract and almost completely converted into the active metabolite ( metabolite ) oseltamivir carboxylate by special liver enzymes (hepatic esterases ) . This active ingredient specifically (selectively) inhibits the neuraminidases of influenza viruses. These neuraminidases are glycoproteins on the surface of a virion (a virus that has not yet penetrated a cell , or viruses that have emerged after entry through replication , which then leave this cell as daughter virions ). They serve the viruses newly formed after replication to dissolve sialic acid , which covers the host cells . The viral neuraminidases inhibited by the metabolite normally have an enzymatic activity that is crucial for the release of newly formed virus particles from infected cells and thus also for the further spread of the infectious virus in the body.
Viruses change the appearance and genetic sequence of their neuraminidase enzyme with each new generation , but a split in this enzyme remains unchanged. It is of essential importance (essential) for the dissolution of sialic acid and is blocked by neuraminidase inhibitors such as zanamivir or oseltamivir.
Oseltamivir therefore reduces the leakage of daughter viruses from the already infected cells and thus the likelihood that the influenza virus will spread further in the body. The preparation can help to shorten the duration of the illness slightly (on average by one day in adults), alleviate the symptoms of the flu and possibly dangerous complications, such as B. pneumonia, can be prevented. However , there is currently no scientific proof of a reduction in mortality through the use of Tamiflu.
Use in therapy
In order to reduce the symptoms of a viral flu, the manufacturer specifies that the drug should be taken as soon as possible after the onset of the symptoms. It is optimal to start treatment within 36 hours of the onset of the first flu symptoms, at the latest after two days. The sooner the therapy begins, the more likely it is that the treatment will be successful.
Side effects
More common adverse effects
Common side effects are nausea , vomiting, and stomach pain. Furthermore, allergic reactions can occur as well as a worsening of existing diseases of the respiratory tract. In order to avoid possible side effects on the gastrointestinal tract, Tamiflu should be taken with some food if possible. There is currently no reliable experience with the active ingredient in the treatment of patients with severe chronic diseases (for example asthma , immune deficiency after operations) or other serious illnesses.
Possible neuropsychiatric incidents in adolescents
In November 2005, reports came out from Japan that inflammation in the brain and significant neuropsychiatric side effects were observed in children and adolescents who had taken Tamiflu (active ingredient: oseltamivir) as a result of an influenza infection. In Japan, therefore, for some time now, clouded awareness, hallucinations and convulsions have also been pointed out as possible side effects.
The US Food and Drug Administration (FDA) initially came to the conclusion that the symptoms were probably due to the underlying disease and not to the drug. An increase in the number of reported cases of neuropsychiatric diseases was known from the 1990s, i.e. before Tamiflu was used. The reason for this increase is, among other things, an increased willingness of Japanese doctors to report such symptoms. After examining more than 100 cases of abnormal behavior, the FDA experts commissioned with the examination in mid-November 2006 also came out in favor of recommending the monitoring of Tamiflu patients on the packaging, even if it was still unclear whether the neuropsychiatric ones were Phenomena can be attributed to the use of the drug or whether these were events that only occurred by chance after taking Tamiflu.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency is also observing reports on possible neuropsychiatric side effects, but confirmed on November 17, 2006 that the authority's statement from the same day of the previous year was still valid. On November 17, 2005, the authorities announced that there was no causal link between Tamiflu and the hallucinations and abnormal behavior described in Japan.
On March 21, 2007, the Japanese Ministry of Health tightened the warnings again. As a precautionary measure, the product information must warn against a prescription for adolescents. At the same time, however, the Japanese ministry re-established that a causal connection between Tamiflu and mental disorders (depression) was not recognizable. In German press reports, Ulrich Hagemann, head of department at the Federal Institute for Drugs and Medical Devices (BfArM), was quoted as saying that there were no reports from the EU about comparable abnormalities in adolescents after taking oseltamivir. Nonetheless, on March 23, 2007, the CHMP recommended: "Patients, especially children and adolescents, should be closely monitored and their caregivers informed immediately if the patient shows signs of unusual behavior."
Drug interactions
Oseltamivir can be taken together with paracetamol , ibuprofen or acetylsalicylic acid (aspirin), the interaction being described as reducing the effectiveness of the medication.
Resistances
The facts presented in the online edition of the respected specialist journal Nature about a certain case of resistance , which is also mentioned in the press again and again (but mostly without specific details), caused quite a stir . According to the publication, a 21-year-old patient in Vietnam was only diagnosed eight days after the onset of the first signs of illness (39.5 ° C fever and cough) and then one day after the diagnosis of severe pneumonia syndrome for a period of seven days with the recommended daily dose of 75 mg twice daily for the treatment of adults so successfully that he was able to leave the hospital afterwards. His 14-year-old sister also became infected (possibly with her brother) and was treated with the prophylactic dose of 75 mg once per day for a total of four days from the second day after the occurrence of mild fever and slight cough . During this therapy, the symptoms of the disease increased, and the patient was therefore given the therapeutic adult dose (2 × 75 mg) for the following seven days . With this dose of active ingredient, the symptoms finally subsided, although researchers found a clear resistance of this virus strain (294S) to oseltamivir in test samples from the fourth day of treatment (of a total of 11) in the pathogen virus found in her and the viral clones made by it. This patient was also able to leave the hospital afterwards.
The authors of the study themselves point out that on the basis of this individual case it is hardly possible to make statements about how quickly a too low dose could lead to the development of resistance in other people after an infection has already occurred, but recommend a close observation of the situation in the sick.
The same genetic modification leading to reduced susceptibility to oseltamivir was detected in early January 2007 in two relatives who died on December 25 and 28, 2006 in Egypt as a result of an H5N1 infection. Both had been treated with oseltamivir to no avail since December 21. The WHO stated on January 18, 2007 that there was no evidence that oseltamivir resistance was widespread in Egypt or anywhere else.
In the 2007/2008 flu season, virus strains resistant to oseltamivir were detected in four European countries. A total of 13% (19 out of 148) of the samples examined had a mutation in vitro that produced resistance. At the end of January 2008, Norwegian doctors discovered an oseltamivir-resistant virus strain ( A / H1N1- H274Y) in normal flu patients, which was then found to be widespread worldwide. For the 2007/2008 flu season in the USA, it was determined on the basis of resistance tests that 12.3% of those infected with H1N1 were resistant to the active ingredient. From the preliminary data for the 2008/2009 flu season, the researchers concluded that around 98% of the H1N1 virus samples they examined were resistant to oseltamivir. WHO data from March 2009 confirm the high level of resistance for the 2008/2009 flu season in 1291 of 1362 samples from 30 countries.
According to the company headquarters of Hoffmann-La Roche in Basel and the Statens Serum Institute (SSI) in Denmark , at the end of June 2009 a patient under treatment with oseltamivir was for the first time oseltamivir-resistant in so-called swine flu (official WHO name : pandemic H1N1 / 09 virus), which is why the patient in question was subsequently treated with the active ingredient zanamivir after discontinuing oseltamivir . Shortly afterwards, isolated resistances were also known from Hong Kong and Japan.
Oseltamivir metabolites are not broken down in sewage treatment plants . During a flu epidemic in Japan, such high concentrations of oseltamivir breakdown products were detected in water that the development of oseltamivir-resistant virus strains in the gastrointestinal tract of water birds is considered possible. Since flu viruses often mutate in birds, there is a possibility that an oseltamivir-resistant flu virus could develop, against which a suitable vaccine would not yet be available.
Use in prophylaxis
The drug can be used to a limited extent for prevention if one or more cases of influenza have already been clearly identified in the environment and contact with the infected person (s) cannot be ruled out. In the technical information from Tamiflu (as of May 2009) it is pointed out, however, that this drug is not a substitute for an influenza vaccination against the known human influenza viruses that have been circulating for years. A use for prevention is only indicated if a vaccination could not previously be carried out for medical reasons for a patient or was not possible or no longer effective at a point in time, such as in a rapidly emerging pandemic .
In response to the possible neuropsychiatric incidents in adolescents reported from Japan, the arznei-telegram 4/2007 recommended : “In view of the marginal benefit in healthy children and adults and the lack of evidence of efficacy in patients with chronic cardiac and / or respiratory diseases, we advise of oseltamivir for virus flu. "
A study on compliance and adverse effects of prophylactic oseltamivir use among 248 English school children was published in July 2009. In view of the frequency and severity of the side effects, 31% complained of a feeling of being very ill, 24% of headache and 21% of stomach pain, it is recommended that prophylactic use be considered after carefully weighing the desired and undesirable effects.
However, there are currently no studies to assess the consequences of taking the drug over longer periods of time (weeks, months). If the drug is used for long-term prophylaxis, the occurrence of additional, serious side effects cannot be ruled out. It is ultimately unclear whether the active ingredient will lower the death rate in a flu epidemic . How useful the use of the active ingredient for long-term prevention in the event of an epidemic could actually be has not yet been clearly determined.
The use of this drug and other possible drugs (such as the dosage) in the treatment and prophylaxis of the flu should be based on current official recommendations and those of the attending physician. New scientific findings on the one hand the effectiveness and side effects of the active ingredients in prevention and therapy also and especially depending on the duration of use and on the other hand a possible development of resistance to these drugs on the pathogen side can be expected at any time. The Robert Koch Institute can also serve as a current source of information .
Measures to prevent an H5N1 pandemic
The World Health Organization (WHO) has advised all states to prevent the influenza pandemic it feared from the avian flu virus H5N1 to keep stocks of this agent in large enough quantities to supply 25% of the population. The flu drug may be useful in bridging the time to develop a vaccine . Antiviral activity against this subtype of the influenza A virus has been demonstrated in laboratory cultures and also in test animals. Due to the small number of sick people, however, clinical studies on the effectiveness of oseltamivir in the H5N1 bird flu are largely lacking.
After France , Norway , Great Britain , Switzerland and the USA , Germany also ordered six million doses of the flu drug in August 2005 . However, critics point out that this number would be far too low in an emergency: Instead of 25% (WHO recommendation) or 20% (Robert Koch Institute) of the population, in some federal states only 10% (Hamburg) or 4 , 5% (Saxony-Anhalt) of the population kept drug doses in stock. In 2006 the Free State of Bavaria alone spent around € 21.9 million on the procurement of drugs against a possible influenza pandemic. According to a company spokesman, the manufacturer is negotiating with the German government about the delivery of more cans. In Baden-Württemberg, Berlin, Hesse, Rhineland-Palatinate or North Rhine-Westphalia, the active ingredient is also stored in sacks and should be filled at short notice if necessary. The federal government still sees Tamiflu as a "suitable means" and bunkers 7.5 million therapy units as a "federal reserve". The French newspaper Liberation reported at the end of August that the French government had already bought five million doses of the flu drug and intended to increase that number to 14 million doses by the end of the year. Tamiflu has a shelf life of 7 years. Roche had earned over eleven billion euros from sales by the end of 2016, an estimated half of which was due to prophylactic storage in the event of a pandemic. Even companies built up emergency supplies for employees.
Since the production of this drug takes a lot of time and is very complex, states and federal states (Lower Austria, Styria, Brandenburg) reserve this product already now [date?] In order to have sufficient supplies in the event of an epidemic.
In the media
Numerous media reports have probably also contributed to the fact that interest in oseltamivir and zanamivir has increased significantly. In the late summer of 2005, more than 130,000 packs of these drugs were sold. In 2004 there were only 30,000 packages in the same period. Combined with the problem of the time-consuming production of active ingredients, this has resulted in Roche restricting deliveries to Germany.
Some media reported that Roche had more or less indirectly promoted this increase in sales. In an article in the ARD magazine Monitor on August 11, 2005, reports on the effectiveness of the drug had been targeted by PR departments to the media. However, this was not done as official advertising, but under the guise of a serious journalistic contribution, which many editorial staff probably had not recognized. Due to some legal disputes, the report has since been removed from the magazine's website as a precaution. The Institute for Health Education (IFGA for short) was usually cited as the source for these journalistic contributions. Obviously, many editorial offices have not questioned this information any further, although such “public relations work” by pharmaceutical companies is well known.
The news magazine Der Spiegel reported on November 19, 2005 that the group was increasingly concerned about its own reputation. Persistent negative reporting damaged the company's image, especially in the USA. In fact, it was previously often criticized in the media that the pharmaceutical company was not or only half-heartedly parting with the exclusive rights to the drug, despite the looming risk of an influenza epidemic. In order to improve its reputation, according to the present report, Roche plans to deepen its cooperation with the PR company Fleishman-Hillard in order to exert “proactive” influence on the media.
Recently, the media have also warned against mass use, because the associated excretion pollutes the water systems in such a way that a considerable environmental impact on the natural microflora and considerable resistance to the virus strains can be expected.
criticism
For several years, the British Medical Journal claims to have tried to gain insight into the raw data on which the optimistic information about the mode of action of oseltamivir was based. It was criticized, among other things, that the names of the developing scientists submitted to the authorities did not match the authors who wrote the publications, which raised the question of a ghostwriter and his competence. In addition, none of the efficacy studies cited by Roche was an independently funded study. After this requirement was not met, the British Medical Journal finally put all correspondence on this matter online in January 2013 as part of its "Open Data Campaign". The Tages-Anzeiger commented on the correspondence as "unmasking": The "detailed documentation" shows that the "health authorities around the globe" had "been satisfied with incomplete documents from the pharmaceutical company" when they had in store "Tamiflu for billions of taxpayers' money" shopping. When the data were finally available, the headline in the Süddeutsche was “Sargnagel für Tamiflu.” Two experts from the Belgian Ministry of Health and the Swedish Institute for Infectious Diseases brought in by the European regulatory authorities have ties to Roche. Experts who previously worked on a study funded by Roche were also involved in drawing up the WHO pandemic guidelines - this study is considered to be the most important work for proving the benefits of Tamiflu. An evaluation of ten studies was mainly written by Roche employees or paid consultants. Even employees of a medical communication agency are said to have worked as ghostwriters on a manuscript for a study on oseltamivir. Statisticians also assumed that some studies had considerable methodological deficiencies.
Web links
- Reto U. Schneider : The race for GS4104 - report on the development of Tamiflu ( NZZ Folio , 01/2004).
- Summary of Evidence for Benefits and Harms of the Use of Influenza Antiviral Agents against Pandemic Influenza A H1N1 (2009) Infections. WHO, November 2010 (PDF; 170 kB)
- Robert Koch Institute: Official Recommendations for Avian Flu Management
- Nike Heinen: The Tamiflu Lie. Less effect, more side effects: published data on flu medication are massively embellished on Sueddeutsche.de (SZ print edition of December 17, 2010)
- Tamiflu has more serious side effects than expected (report on side effects on Welt.de from January 18, 2012)
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Oseltamivir
Individual evidence
- ↑ a b c d e data sheet Oseltamivir phosphate, ≥98% (HPLC) from Sigma-Aldrich , accessed on October 31, 2016 ( PDF ).
- ↑ a b H. J. Schünemann, SR Hill, M. Kakad et al .: WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus . In: Lancet Infect Dis . 7, No. 1, January 2007, pp. 21-31. doi : 10.1016 / S1473-3099 (06) 70684-3 . PMID 17182341 .
- ↑ a b c Tom Jefferson, Mark A Jones, Peter Doshi et al .: Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. In: British Medical Journal . 2014, online pre-publication of April 10, 2014, doi: 10.1002 / 14651858.CD008965.pub4 .
- ↑ Zosia Kmietowicz: WHO downgrades oseltamivir on drugs list after reviewing evidence , online June 12, 2017, accessed July 1, 2019
- ↑ arznei-telegram (PDF; 28 kB).
- ↑ Record result: highest profit in the company's history for Roche. In: tagesspiegel.de . Retrieved November 13, 2018 .
- ↑ n-tv news: Roche sticks to its outlook. In: n-tv.de. April 17, 2008, accessed November 13, 2018 .
- ^ Roche: Half-year report 2011 . (PDF file)
- ^ Roche: Half-year report 2018 . (PDF file)
- ↑ eurekalert.org of January 17, 2012: Continuing uncertainties surround anti-influenza drug.
- ↑ eurekalert.org of January 17, 2012: Effects of Tamiflu still uncertain, warn experts, as Roche continues to withhold key trial data.
- ↑ nature.com of January 13, 2012: Researchers renew fight with Roche over flu drug evidence.
- ↑ welt.de of January 18, 2012: Tamiflu has more serious side effects than expected.
- ↑ T. Jefferson, V. Demicheli, D. Rivetti, M. Jones, C. Di Pietrantonj, A. Rivetti: Antivirals for influenza in healthy adults: systematic review. In: Lancet. Volume 367, number 9507, January 2006, pp. 303-313, doi: 10.1016 / S0140-6736 (06) 67970-1 , PMID 16443037 (review).
- ↑ Toshihiro Tanaka et al .: Safety of neuraminidase inhibitors against novel influenza A (H1N1) in pregnant and breastfeeding women. In: Canadian Medical Association Journal. 2009, Volume 181, No. 1-2, pp. 55-58, doi: 10.1503 / cmaj.090866 .
- ↑ Ärzteblatt.de: FDA: Tamiflu is also safe for children under 1 year of age.
- ↑ Hoffmann-La Roche : Factsheet Tamiflu ( Memento from February 22, 2016 in the Internet Archive ) (PDF; 336 kB), as of November 17, 2006.
- ↑ Japanese researcher finds synthetic route to Tamiflu. Reported to Nature on March 8, 2006.
- ↑ Mita T, Fukuda N, Roca FX, Kanai M, Shibasaki M: Second generation catalytic asymmetric synthesis of Tamiflu: allylic substitution route . In: Org. Lett. . 9, No. 2, January 2007, pp. 259-262. doi : 10.1021 / ol062663c . PMID 17217279 .
- ↑ Satoh N, Akiba T, Yokoshima S, Fukuyama T: A practical synthesis of (-) - oseltamivir . (PDF) In: Angew. Chem. Int. Ed. Engl . 46, No. 30, 2007, pp. 5734-5736. doi : 10.1002 / anie.200701754 . PMID 17594704 .
- ↑ Trost BM, Zhang T: A concise synthesis of (-) - oseltamivir . In: Angew. Chem. Int. Ed. Engl . 47, No. 20, 2008, pp. 3759-3761. doi : 10.1002 / anie.200800282 . PMID 18399551 .
- ↑ a b A Short Enantioselective Pathway for the Synthesis of the Anti-Influenza Neuramidase Inhibitor Oseltamivir from 1,3-Butadiene and Acrylic Acid Ying-Yeung Yeung, Sungwoo Hong, and EJ Corey J. Am. Chem. Soc. ; 2006 ; 128 (19) pp. 6310-6311; (Communication) doi: 10.1021 / ja0616433 .
- ^ Research groups in the US and Japan develop routes that avoid shikimic acid . In: Chemical & Engineering News . 2006, Volume 84, No. 18, p. 5.
- ↑ What use are neuraminidase inhibitors in a flu pandemic? In: arznei-telegram. ATI, Berlin 36, 2005, No. 7, pp. 62f. ISSN 0066-8192 .
- ↑ Press release ( memento of October 8, 2009 in the Internet Archive ) (pdf; 105 kB).
- ↑ European Medicines Agency update on the safety of Tamiflu (emea) ( Memento of October 8, 2009 in the Internet Archive ) of November 2005 (pdf; 100 kB).
- ↑ www.handelsblatt.com and various agency reports from March 22, 2007.
- ^ Message from the afp agency dated March 22, 2007, 4:44 p.m.
- ↑ www.ema.europa.eu ( Memento of October 8, 2009 in the Internet Archive ) (PDF; 30 kB) Press release of March 23, 2007.
- ↑ Le Qm et al. : Avian flu, isolation of drug-resistant H5N1 virus. In: Nature . London 2005, Volume 437, No. 7062, October 20, p. 1108, PMID 16228009 .
- ↑ WHO's Epidemic and Pandemic Alert and Response: "At this time there is no indication that oseltamivir resistance is widespread in Egypt or elsewhere." Of January 18, 2007.
- ↑ Robert Koch Institute: Influenza: On the occurrence of resistance to oseltamivir in influenza viruses of the subtype A / H1N1. from February 1, 2008.
- ↑ Resistance to oseltamivir (Tamiflu) in some influenza A (H1N1) virus samples. ECDC, September 20, 2008.
- ↑ Influenza A (H1N1) virus resistance to oseltamivir - 2008 influenza season, southern hemisphere. ( Memento of April 2, 2015 in the Internet Archive ) (PDF; 33 kB) WHO, August 20, 2008.
- ↑ NJ Dharan, LV Gubareva, JJ Meyer et al. : Infections With Oseltamivir-Resistant Influenza A (H1N1) Virus in the United States. In: JAMA. March 2, 2009, Volume 301, No. 10, doi: 10.1001 / jama.2009.294 .
- ↑ Influenza A (H1N1) virus resistance to oseltamivir - 2008/2009 influenza season, northern hemisphere. (PDF; 39 kB) WHO, March 18, 2009.
- ↑ who.int (PDF; 70 kB) of July 7, 2009: Transcript of virtual press conference with Dr. Keiji Fukuda, Assistant Director-General ad Interim for Health Security and Environment, World Health Organization .
- ↑ taz.de "Better to be ill than dead", Danger from the Tami River, TAZ, July 24, 2009.
- ↑ arznei-telegram 2007; 38:40 Retrieved March 18, 2020.
- ↑ A. Wallenstein et al. : Compliance and side effects of prophylactic oseltamivir treatment in a school in South West England. In: Eurosurveillance . Volume 14, No. 30, 2009, full text .
- ↑ Science, August 5, 2005, p. 871.
- ^ Netzeitung ( Memento from May 1, 2012 in the Internet Archive ).
- ↑ Bavarian Supreme Court of Auditors: Annual Report 2008, part no. 8.1, p. 20 (PDF; 738 kB) .
- ↑ Martin U. Müller : Influenza drug Tamiflu: The madness about a supposed miracle drug . In: Der Spiegel . August 14, 2017 ( spiegel.de [accessed February 19, 2018]).
- ↑ European Medicines Agency recommendations on extension of shelf life for Tamiflu Declaration of the European Medicines Agency dated May 8, 2009.
- ↑ Martin U. Müller: Influenza drug Tamiflu: The madness about a supposed miracle drug . In: Der Spiegel . August 14, 2017 ( spiegel.de [accessed February 19, 2018]).
- ↑ Andrew Singer, Oxford University, based on Der Spiegel 2007, 5, 130.
- ↑ Tamiflu: the battle for secret drug data In: British Medical Journal. 2012, Volume 345, e7303, doi: 10.1136 / bmj.e7303 .
- ↑ a b F. Godlee, M. Clarke: Why do not we have all the evidence on oseltamivir ?. In: BMJ. Volume 339, 2009, pp. B5351-b5351, doi: 10.1136 / bmj.b5351 .
-
↑ Tamiflu correspondence with Roche
Tamiflu correspondence with the World Health Organization
Tamiflu correspondence with the Centers for Disease Control and Prevention
Correspondence with the European Medicines Agency . - ↑ Doubts about Tamiflu - The pressure on Roche is increasing. In: Tages-Anzeiger . January 26, 2013 (viewed online January 28, 2013).
- ^ Coffin nail for Tamiflu In: Süddeutsche . April 10, 2014 (online April 3, 2016).
- ↑ Martin U. Müller : Influenza drug Tamiflu: The madness about a supposed miracle drug . In: Der Spiegel . August 14, 2017 ( spiegel.de [accessed February 19, 2018]).
