Zanamivir
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
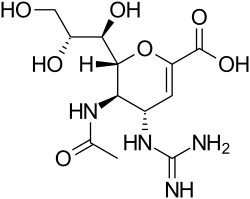
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Zanamivir | |||||||||||||||||||||
| other names |
(4 S , 5 R , 6 R ) -5-acetylamino-4-guanidino-6 - [(1 R , 2 R ) -1,2,3-trihydroxypropyl] -5,6-dihydro-4 H -pyran- 2-carboxylic acid |
|||||||||||||||||||||
| Molecular formula | C 12 H 20 N 4 O 7 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Inhibition of viral neuraminidase, inhibition of the release of newly formed influenza A and B viruses |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 332.31 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
256 ° C (zanamivir sesqui hydrate ) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Zanamivir is the first drug from the group of neuraminidase inhibitors that is used to treat the flu (influenza) . The drug Relenza ® was developed by Biota Holdings , Australia ; it has been marketed by GlaxoSmithKline since 1999 and requires a doctor's prescription .
state of research
As an inhibitor of viral neuraminidase , it can prevent the release of further viruses and thus the occurrence or progression of the disease, provided it is administered within the first 48 hours. In 2014, research by the Cochrane Collaboration revealed doubts about the efficacy and safety of zanamivir and oseltamivir . A meta-analysis by the Cochrane Collaboration found that treatment with zanamivir in adults reduced the duration of illness from 6.6 to 6 days. However, zanamivir had no effect on the incidence of severe forms of disease such as pneumonia or bronchitis.
application
Since zanamivir only about 2% from the gastrointestinal tract is received, it must be as a powder using the inhaler Diskhaler be administered.
Studies
therapy
The first clinical studies with zanamivir for the treatment of infections with influenza viruses took place in 1994/1995.
In young patients with an average age of 30 years with proven influenza infection, the duration of the fever was reduced from 6.8 days when given placebo to 5.2 days when given zanamivir, provided that it was taken within 30 hours of the onset of symptoms. The time to clinical improvement / freedom from symptoms was 6.3 days in the placebo group compared to 5.4 days in the zanamivir group.
Other studies largely confirmed these results and attested zanamivir a reduction in the duration of fever from 6 days on placebo to 4.5 days or a reduction in the duration of symptom-free treatment by one day.
In Germany, the Federal Committee of Doctors and Health Insurance Companies declared the drug dispensable in 2000.
prophylaxis
A randomized controlled trial showed at 1,107 students during a flu - epidemic that daily administration of Relenza over a period of 28 days resulted in a decrease in influenza disease by 6% in the placebo group to 2% in the Relenzagruppe: In the 34 of 554 study participants in the placebo group and 11 of 553 study participants in the zanamivir group. 19 people in the placebo group and 3 in the zanamivir group suffered from fever. This means that for every 35 people treated, one influenza could be prevented.
Side effects
The most common adverse drug reactions in people with bronchial asthma or chronic obstructive pulmonary disease are more than 20% reduction in lung function (FEV1 or peak flow ). Sometimes severe bronchospasm with isolated deaths occurred here .
discussion
Data on the efficacy and safety of this substance in elderly patients and risk groups are just as little available as clear data on the effect on the incidence of complications and deaths.
There is still little data available on the use of zanamivir in patients with impaired renal or hepatic function and in heart failure .
Manufacturing
Various multi-step syntheses for zanamivir, starting from N- acetyl neuraminic acid , are described in the literature.
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1744, ISBN 978-0-911910-00-1 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of zanamivir in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on July 12, 2020, is reproduced from a self-classification by the distributor .
- ↑ a b c Tom Jefferson, Mark A Jones, Peter Doshi, Chris B Del Mar, Rokuro Hama, Matthew J Thompson, Elizabeth A Spencer, Igho Onakpoya, Kamal R Mahtani, David Nunan, Jeremy Howick, Carl J Heneghan: Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. In: British Medical Journal (2014), online pre-publication. doi : 10.1002 / 14651858.CD008965.pub4 .
- ↑ Monto AS, Robinson DP, Herlocher ML et al. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999 Jul 7; 282 (1): 31-5, PMID 10404908 . Full text , accessed September 26, 2008.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances , 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.