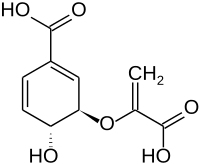
Chorismic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chorismic acid | |||||||||||||||
| other names |
(3 R , 4 R ) -3- (1-carboxy-4-hydroxy-cyclohexa-1,5-dienyl) oxyisoprop-2-enoic acid |
|||||||||||||||
| Molecular formula | C 10 H 10 O 6 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 226.18 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chorismic acid is the common name for (3 R , 4 R ) - (1-carboxyvinyloxy) -4-hydroxy-1,5-cyclohexadiene-1-carboxylic acid. This carboxylic acid is an important intermediate in the biosynthesis of the human essential aromatic amino acids tyrosine , tryptophan and phenylalanine in plants , bacteria and fungi in the shikimic acid pathway . Their salts are called chorismates . The name chorismate comes from the Greek (from Greek χωρίζομαι to separate) and means “branch”, since the biosynthetic path here branches either via prephenic acid in the direction of tyrosine and phenylalanine or in the direction of tryptophan.
The conversion of chorismic acid to prephenic acid takes place by a Claisen rearrangement , which is catalyzed by the enzyme chorismate mutase ( EC 5.4.99.5 ).
In addition, in an alternative synthesis route, salicylic acid is formed from isochorismic acid in plants , which in turn is formed from chorismate by isochorismate synthase .
Individual evidence
- ↑ a b Datasheet Chorismic acid from Enterobacter aerogenes from Sigma-Aldrich , accessed on March 22, 2011 ( PDF ).
- ↑ Entry on prephenic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ^ Hans Walter Heldt, Birgit Piechulla: Plant biochemistry . 5th edition. Springer, Berlin, Heidelberg 2015, ISBN 978-3-662-44398-9 , pp. 418 , doi : 10.1007 / 978-3-662-44398-9 .
Web links
- Directed evolution
- Catalytic Antibodies ( Memento from February 27, 2012 in the Internet Archive )
- Shikimic acid biosynthesis

