Oliving group
| Oliving group | |
|---|---|
| Olivine from Mount Erebus , Ross Island, Antarctica | |
| General and classification | |
| other names |
Olivine |
| chemical formula | (Mg, Mn, Fe) 2 [SiO 4 ] |
|
Mineral class (and possibly department) |
Island silicates (nesosilicates) |
|
System no. to Strunz and to Dana |
9.AC.05 ( 8th edition : VIII / A.04) 51.03.01.00 |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | orthorhombic-dipyramidal; 2 / m 2 / m 2 / m |
| Frequent crystal faces | {110}, {120} in combination with {021}, {101}, {111} etc. {010} |
| Physical Properties | |
| Mohs hardness | 6.5 to 7 |
| Density (g / cm 3 ) | 3.2 to 4.4 |
| Cleavage | good after {001}, clearly after {010} |
| Break ; Tenacity | shell-like, brittle |
| colour | light green to dark green, yellow brown to black |
| Line color | White |
| transparency | transparent to translucent |
| shine | Glass gloss to fat gloss |
| Crystal optics | |
| Refractive indices |
n α = 1.630 to 1.650 n β = 1.650 to 1.670 n γ = 1.670 to 1.690 |
| Birefringence | δ = 0.040 |
| Optical character | biaxial positive |
The olivine group (or olivine for short ) is a group of minerals with a similar composition from the mineral class of " silicates and germanates ". By definition, this group includes island silicates with the general composition A 2 2+ [SiO 4 ], where A serves as a placeholder for the chemical elements lead , calcium , cobalt , iron , magnesium , manganese and nickel .
All olivines crystallize in the orthorhombic crystal system (the only exception is Laihunit ) and develop mostly transparent to translucent crystals with a strong glass luster and a tabular to prismatic habit . The color usually varies between light and dark green, but can also be yellow-brown to black. However, olivine always leaves a white line on the marking board .
Etymology and history
The abbreviation olivine comes from the Latin oliva for olive . Abraham Gottlob Werner chose this name in 1790 because of the predominantly olive to bottle green color of this mineral group.
The gemstone variety peridot has been used since the 15th century BC. Chr. On the island Zebirget (Zabargad) in the Red Sea dismantled. He became known in Europe mainly through the Crusades . It was not until 1772 that normal olivines were recognized as independent minerals - of all things, in a meteorite .
classification
In the now outdated, but still in use, 8th edition of the mineral classification according to Strunz , the olive group belonged to the general department of "island silicates (nesosilicates)", bearing the system no. VIII / A.04 and consisted of the members Fayalit , Forsterit , Laihunit , Liebenbergit and Tephroit .
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), also classifies the olive group in the category of "island silicates (nesosilicates)". However, this is further subdivided according to the possible presence of further anions and the coordination of the cations involved , so that the oliving group with system no. 9.AC.05 according to the composition of the members Fayalite, Forsterite, Glaukochroit , Kirschsteinite , Laihunite, Liebenbergite and Tephroit in the sub-section “Island silicates without further anions; Cations in octahedral [6] coordination ”.
The Oliving group also classifies the mineral system according to Dana , which is mainly used in the English-speaking world, in the “island silicate minerals ” section. Here it bears the system no. 51.03.01 , consists of the members olivine, fayalite, forsterite, liebenbergite, tephroit and laihunite and can be found in the sub-section “ Island silicates: SiO 4 groups with all cations only in octahedral [6] coordination ”.
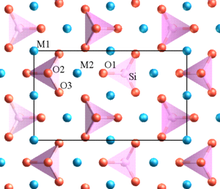
Crystal structure
The structure of the olivine is similar to that of a hexagonal closest packing of spheres , in which the oxygen atoms represent the packing planes. The silicon fills the resulting tetrahedral gaps , while magnesium and iron fill the octahedral gaps accordingly .
In the upper mantle, olivines undergo two phase changes due to the increasing pressure and temperature . At a depth of about 410 km ( 410 km discontinuity ) the high pressure modification wadsleyite ("modified spinel", often referred to as β) occurs and from a depth of about 520 km ( 520 km discontinuity ) it turns into ringwoodite (" Spinel ”, often referred to as γ) over.
The term "spinel" only refers to the crystal structure and should not be confused with the actual mineral spinel . At the boundary between the upper and lower mantle at a depth of 660 km, ringwoodite finally breaks down into bridgmanite (Mg, Fe) SiO 3 ( perovskite structure) and magnesiowustite (Mg, Fe) O. In particular, the phase boundaries at 410 and 660 km are associated with striking seismic discontinuities at which earthquake waves are reflected or refracted, and thus define the mantle transition zone .
Single minerals and varieties
In the narrower sense, olivine is predominantly seen as a mixed crystal of the forsterite – fayalite series, but these minerals also form mixed crystals with tephroite, so that the olivine series actually consists of three end links:
- the denser iron-containing fayalite Fe 2 [SiO 4 ] ( molar mass 203.78; melting point 1490 K )
- the magnesium-containing forsterite Mg 2 SiO 4 (molar mass 140.71; melting point 2163 K)
- the manganese-containing Tephroit Mn 2 SiO 4
Named intermediate links are hyalosiderite and hortonolite , which are not, however, independent minerals. Clear and large olivine crystals are valued gemstones and are known as peridot or chrysolite.
Education and Locations
Olivines are the most common silicates and rock-forming minerals. They form the main component of the upper mantle , where the magnesium and iron components of olivine are in a ratio of about 9: 1, and arise in basic and ultra-basic intrusive igneous rocks such as gabbro and peridotite , but also in extrusive ones such as basalt . Dunite is an intrusive rock that consists almost exclusively of olivine and in which forsterite crystals up to 15 cm in size have been found.
By metamorphism olivine arises as forsterite from dolomite-rich limestone ; conversely, through weathering processes and through contact with mineral-rich hydrothermal solutions, serpentines are formed from olivine (serpentinization). The erosion of basalt lava leads to the formation of dark green olivine sands in some places. Finally, olivine also occurs in a group of stone-iron meteorites , the pallasites and most chondrites, as well as some stone meteorites such as the ureilites . The olivine crystals are embedded in a nickel - iron matrix.
At the beginning of March 2007 it was reported that in the area of the Fifteen-Twenty Fracture Zone (FTFZ) of the Mid-Atlantic Ridge , halfway between Barbados and Tenerife , an unusual hole was discovered in the earth's crust, through which one can directly access the rock of the earth's mantle from green shimmering olivine.
In April 2011, an American research team reported the discovery of olivine crystals ( forsterite ) in the protostellar cloud of the protostar HOPS-68 using the Spitzer space telescope . The scientists assume that the initially amorphous material is tempered near the protostar and crystallizes in the process, before it is transported to the cooler outer area of the dust envelope by transport processes. Olivine has also been detected in numerous other cosmic environments using methods of infrared spectroscopy: in several comets, in red giant stars pulsing in dust envelopes, in planetary nebulae and in protoplanetary disks.
use
The particularly pure, transparent-green variant of olivine, peridot and chrysolite, are used as gemstones . Ordinary olivine is used in the manufacture of heat-resistant glass and refractory materials , as well as in the manufacture of iron ore pellets as a slag former. Olivine sand is used as molding sand in metal foundries and as an abrasive . It also serves as a catalyst in wood gasification processes , for example in the pilot plant in Güssing , Austria.
Olivine is also suitable as a heat store, for example in night storage heaters, and also very well as an infusion stone for the sauna .
See also
literature
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th, revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 454-460 .
- Helmut Schrätze, Karl-Ludwig Weiner: Mineralogy. A textbook on a systematic basis . de Gruyter, Berlin, New York 1981, ISBN 3-11-006823-0 , pp. 654 .
- Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
Web links
- Mineral Atlas: Olivine (Wiki)
- Jürgen Kühnle: Mineral Lexicon - Olivine. In: Wissen-im-netz.info. Knowledge on the Net, archived from the original on March 5, 2016 ; accessed on January 22, 2019 .
- GeoLexicon - Olivine. In: geodz.com. GeoDataZone, May 27, 2018, accessed January 22, 2019 .
- Michael RW Peters: peridot, forsterite and dunilite. Varieties of the mineral olivine. In: realgems.org. RealGems, November 30, 2016, accessed on January 22, 2019 (with images of cut olivine or peridot, forsterite, dunilite and pallasite).
- Olivine. In: mindat.org. Hudson Institute of Mineralogy, accessed January 22, 2019 .
Individual evidence
- ↑ Hans Jürgen Rösler : Textbook of Mineralogy . 4th, revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 454 .
- ↑ Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . 2nd Edition. Ott Verlag, Thun 1979, ISBN 3-7225-6265-1 , p. 285 .
- ↑ Martin Okrusch, Siegfried Matthes: Mineralogie. An introduction to special mineralogy, petrology and geology . 7th, completely revised and updated edition. Springer, Berlin [a. a.] 2005, ISBN 3-540-23812-3 , pp. 420-421 .
- ↑ Hans Jürgen Rösler : Textbook of Mineralogy . 4th, revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 455 .
- ↑ Markus Becker: Researchers are studying a huge hole in the earth's crust. In: spiegel.de. Der Spiegel, March 6, 2007, accessed on January 22, 2019 .
- ↑ Drilling the Mid-Atlantic Ridge: RRS James Cook cruise JC007, March 5, 2007 - April 17, 2007. In: classroomatsea.net. Classroom @ Sea, archived from the original on March 4, 2016 ; accessed on January 22, 2019 .
- ^ Charles A. Poteet, S. Thomas Megeath, Dan M. Watson, Nuria Calvet, Ian S. Remming, Melissa K. McClure, Benjamin A. Sargent, William J. Fischer, Elise Furlan, Lori E. Allen, Jon E. Bjorkman, Lee Hartmann, James Muzerolle, John J. Tobin, Babar Ali: A Spitzer-IRS Detection of Crystalline Silicates in a Protostellar Envelope . In: Astrophysical Journal Letters . tape 733 , no. 2 , May 10, 2011, doi : 10.1088 / 2041-8205 / 733/2 / L32 , arxiv : 1104.4498v1 (English, arxiv.org [PDF; 277 kB ; accessed on January 22, 2019]).
- ↑ Thomas Henning: Astromineralogy . 2nd Edition. Springer, Berlin, Heidelberg 2010, ISBN 978-3-642-13258-2 .
- ^ W. Pohl: Mineral and energy raw materials . 5th edition. Schweizerbart, Stuttgart 2005, ISBN 3-510-65212-6 , p. 273 .