Silicates

Silicates (also known as silicates ) are the salts and esters of orthosilicic acid (Si (OH) 4 ) and their condensates . The esters are described under silicic acid esters , for the condensates see silicas . All the salts are characterized by SiO 4 - tetrahedron built compounds whose tetrahedra may be linked to each other but in different ways. Unlinked parts of the tetrahedron contribute to the charge balance as metal cations or may be present as hydroxide ions (OH - ). With the exception of alkali silicates , silicates are insoluble in water or other solvents .
Natural silicates (silicate minerals ) play a major role in mineralogy , as a large number of minerals can be assigned to this group of substances. The earth's crust consists of over 90 percent and the earth's mantle almost entirely of silicates. The most common silicates in the earth's crust are feldspars with 50–60 percent by volume . Other important rock-forming minerals are mica , clay minerals , amphiboles , pyroxenes , garnet and olivine . The common mineral quartz (SiO 2 ) is counted among the oxides in German-language literature , but among the silicates in Anglo-American literature.
structure
All silicate minerals have a common construction principle, which is why they can be arranged relatively easily in a systematic order. The basic building blocks of all silicates are SiO 4 - tetrahedrons . One silicon atom is surrounded by four oxygen atoms. The oxygen atoms touch each other because of their size, there is room in the middle for the relatively small silicon atom (the free space is called the tetrahedral gap ).
Another property of the silicates is the ability of the oxygen atoms to participate in different SiO 4 complexes at the same time . In addition to isolated SiO 4 tetrahedra, this results in other composite components:
- isolated tetrahedron
- Double tetrahedron
- Ring structures
- Single and double chains
- Layer structures
- Framework structures.
Aluminum can replace and substitute silicon, which behaves chemically in a similar way (one speaks here of “isomorphic replacement”), silicates in which this happens are called aluminosilicates . When aluminum (Al 3+ instead of Si 4+ ) is installed in the mineral lattice, the charge must be balanced by installing additional positively charged ions ( cations ). The Al: Si ratio cannot exceed 1, pure aluminates do not occur in nature.
Systematics of the silicate minerals
As already mentioned, the silicates form an extremely extensive mineral family. There are great differences in chemical composition, crystal symmetry , types of bond and structure of the basic building blocks, which is why there are different classification schemes for silicate minerals. The system used in Germany divides them according to the degree of polymerization of the SiO 4 tetrahedra.
Comments on the notation of chemical molecular formulas
A simplified schematic formula of silicates is:
- .
The oxygen - silicon complexes can be replaced by hydroxide or fluoride ions . The position of "M" is occupied by one or more metal ions until the charge is equalized. Water can also be stored in the lattice of particularly wide-meshed silicates . If some of the Si x O y complexes in a certain mineral are actually replaced by ions such as fluoride (F - ) or hydroxide (OH - ), this is indicated by vertical dividing lines in the last term of the formula, for example
- , Kaolinite .
Stored water is noted as follows:
- , Analcime .
Classification according to the degree of polymerization of the SiO 4 tetrahedra
Island silicates (nesosilicates)
The island silicates have isolated SiO 4 tetrahedra. Representative:
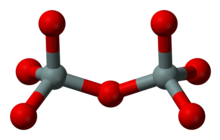
Group silicates (sorosilicates)
Every two SiO 4 complexes are linked to form double tetrahedra via an oxygen atom, with half of this so-called bridging oxygen being part of each SiO 4 tetrahedron. The Si: O ratio in group silicates is therefore 2: 7. This structure is less common; an example is the mineral gehlenite (Ca 2 Al [(Si, Al) 2 O 7 ]).
Ring silicates (cyclosilicates)
In ring silicates , the SiO 4 tetrahedra are grouped into isolated rings of three, four and six. Each silicon ion shares two oxygen ions with two neighboring tetrahedra, resulting in an Si: O ratio in ring silicates of 1: 3. The following formulas result for the ring structures:
- [Si 3 O 9 ] 6−
- [Si 4 O 12 ] 8−
- [Si 6 O 18 ] 12− .
Beryl (Al 2 Be 3 [Si 6 O 18 ]) and the minerals of the tourmaline group belong to the ring silicates.
Chain and band silicates (inosilicates)
The chain silicates belong to two important groups of rock-forming minerals: pyroxenes and amphiboles . The pyroxenes form one-dimensional single chains, two of the oxygen ions belong to two tetrahedron complexes at the same time, resulting in a Si: O ratio of 1: 3, as with diopside (CaMg [Si 2 O 6 ]).
Amphiboles form one-dimensional bands. Two chains are connected laterally via bridging oxygen. Compared to the chains, every second tetrahedron of each chain has an oxygen ion in common with its respective neighbor. The Si: O ratio in strip silicates is 4:11. In such siliceous bands voids are present in the (OH) - - and F - ions can occur. In the chemical formula, this is expressed by a vertical line. A mineral from the amphibole group is actinolite (Ca 2 (Mg, Fe) 5 [(OH) 2 | Si 8 O 22 ]).
Layered silicates (phyllosilicates)
With a higher degree of polymerization, layer structures made of SiO 4 tetrahedra form instead of chain structures . Within a layer, each silicon atom shares three of its oxygen ions with its neighbors. The Si: O ratio of the layered silicates is 2: 5. The layered silicates are divided into two- and three-layered silicates. Another subdivision takes into account the structure and the ions that are between two tetrahedral layers. The cavity between two layers can be occupied by (-OH), (-O - Me + ) and connect the layers with dipole-dipole forces or ionic bonds .
The sheet silicates include mineral groups such as mica , talc , serpentine and clay minerals such as vermiculite , examples are muscovite (a three- layer silicate ) (KAl 2 [(OH) 2 | AlSi 3 O 10 ]) and kaolinite (a two-layer silicate) (Al 4 [(OH ) 8 | Si 4 O 10 ]).
Synthetic sheet silicates such as SKS-6 (Na 2 Si 2 O 5 ) are used in detergents . SKS-6 sheet silicates show properties like sodium zeolites . The layer-connecting, hydrated sodium ions are selectively exchangeable in suspensions , for example for calcium ions, and are therefore suitable for water softening as ion exchangers and have good properties as washing alkalis .
Framework silicates (tectosilicates)
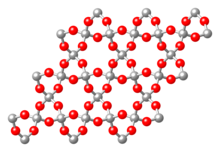
In the case of framework silicates, each oxygen ion belongs to two neighboring tetrahedra at the same time (each tetrahedron is connected to neighboring tetrahedra via its corners). This creates three-dimensional network structures. The result is the chemical formula SiO 2 . The quartz shown here in the β-quartz modification is representative of silicon dioxide compounds . For further framework silicates, silicon has to be replaced by aluminum. The charge balance takes place through the incorporation of cations . The framework silicates include the feldspars and feldspar representatives , an extremely important group of minerals because of their abundance. Examples are minerals from the mixed series of plagioclase (albite - anorthite): (NaAlSi 3 O 8 - CaAl 2 Si 2 O 8 ).
Even large molecules such as H 2 O can be incorporated into the wide-meshed grid of some feldspar representatives . At high temperatures the water escapes, but at low temperatures it is re-incorporated into the crystal lattice in an environment saturated with water vapor . These water-containing minerals belong to the group of zeolites (e.g. natrolite (Na 2 [Al 2 Si 3 O 10 ] · nH 2 O)).
Amorphous silicates
Opal is amorphous silicon dioxide with embedded water (SiO 2 · n H 2 O). Like quartz, some authors place it among the oxide minerals . The highly structured shells of diatoms (diatom) and Strahlentierchen (Radiolaria) are of amorphous silica (SiO 2 established).
Classification according to Kostov
This classification is mainly based on the chemical composition of the silicate and its crystal morphology.
Technical silicates
- Many silicates are manufactured industrially. The most prominent places here are the glasses and glass ceramics , but also zirconium silicates as color pigments. These are usually made from quartz sand (SiO 2 ) and metal oxides in a glass melting furnace .
- Glasses that are soluble in water, so-called water glasses , are made from quartz sand and metal carbonates (with soda Na 2 CO 3 ) in a glass melting furnace. They are used as adhesives , fillers in the paper industry , for sealing damp masonry , and as additives in vapor - permeable plaster mixtures . Gels ( silica gel ), silicic acid , silicates and zeolites are produced on an industrial scale from water glass by reaction with acids .
- Talk can be used in many ways. It is used in the paint and glass industry and as a lubricant. As a ground raw material (then called talc ) it is contained in many cosmetics.
- Asbestos ( chrysotile ) was used due to its fire resistance and its suitability as an insulating and insulating material, especially in the construction industry, but has been banned in the EU since 2005 because of its harmful side effects. The minerals zircon, muscovite , andalusite , sillimanite and thistle are also suitable for the production of refractory and corrosion-resistant materials .
- Kaolinite is an important raw material for the ceramic industry for the manufacture of refractory crucibles and wall and roof tiles.

- Zeolites, especially the synthetic zeolite A , are used as ion exchangers (so-called molecular sieves) and serve as a phosphate substitute to soften water , especially in detergents . To prevent eutrophication of ornamental ponds , nutrients ( ammonium compounds ) are removed with zeolites and, in particular, the growth of algae is inhibited. In addition to these aluminosilicates , synthetic sheet silicates ( see above ) with similar properties were found.
- Because of their large surface area and adsorption capacity, nanosilicates are suitable as carriers for catalyst materials or medicinal agents. They can be produced, for example, by the Stöber synthesis or on an industrial scale as pyrogenic silicon dioxide ( Aerosil ).
- Silicates are widely used as corrosion inhibitors in drinking water treatment . Phosphate-silicate mixtures and phosphate-free, carbonate-activated silicates are known to be effective inhibitors.
Occurrence
- Silicates are found in dissolved form in all water in low concentrations.
- Some groups of organisms form pebbly skeletons, the main production is probably done by planktonically living organisms such as diatoms and radiolarians . Some sponges also build up pebble framework structures.
- All earth-like planets consist to a large extent of silicates.
use
As jewelry and precious stones
- The island silicates garnet , olivine , topaz and zircon are sold in pure quality as gemstones .
- The quartz varieties amethyst , aventurine quartz , chalcedony , citrine and moss agate are popular gemstones.
- The feldspar amazonite , aventurine feldspar , labradorite and the feldspar representative sodalite are used as gemstones.
- The gemstones emerald and aquamarine are a variety of beryl .
Industry
- in silicate glass ( building glass )
- In borosilicate glass ( laboratory glass ), also as a material for kitchenware made of glass ( Duran , Jenaer Glas , Pyrex ) or as a display cover for technical devices ( Willow Glass )
- in aluminosilicate glass (see alu mosilicate ) as glass with high fire resistance (according to DIN EN 1059) and as a scratch-resistant display cover for technical devices ( Gorilla Glass )
- in lead glass
- in undoped and doped silicon oxide layers, often referred to here as silicate glass, e.g. B. borophosphorus silicate glass , a silicate glass doped with 3 to 5% boron and phosphorus, which is used in semiconductor technology, among other things, to planarize the surface
- in the detergent industry
- as a highly absorbent, odor-neutralizing cat litter
- as fire protection panels
- in colors
proof
Silicate can be detected with the water drop sample. Calcium fluoride and sulfuric acid are added to the substance in a lead crucible . In the presence of silicon dioxide, gaseous silicon tetrafluoride is formed , which condenses again to white silicon dioxide on a moistened, black filter paper above the crucible opening
- Fluoride anions react with sulfuric acid to form sulfate anions and hydrogen fluoride .
- Silicon dioxide reacts with hydrogen fluoride to form silicon tetrafluoride and water. The forward reaction takes place at the bottom of the crucible, the reverse reaction at the top of the lid.
literature
- WL Bragg : The structure of silicates. In: Z. Kristallogr. 74. 1930, 237-305.
- WA Deer, WA Howie, and J. Zussman: Rock-Forming Minerals, Volume 1A: Orthosilicates . Longman, London, 2nd edition, 1982.
- I. Kostov: Crystal chemistry and classification of silicate minerals. In: Geokhimiya, Mineralogiya i Petrologiya. 1. 1975, 5-41.
- F. Liebau: The systematics of silicates. In: Natural Sciences. 49. 1962, 481-491.
- F. Liebau: Structural Chemistry of Silicates . Springer-Verlag, Berlin 1985.
- S. Matthes: Mineralogy . Springer-Verlag, Berlin, 4th edition, 1993 (there is now a new edition, date unknown)
- S. Na'ray-Szabo: A silicate system based on the crystal structure. In: Z. Physics. Chem. Dept. B9. 1930, 356-377.
- H. Pichler and C. Schmitt-Riegraf: Rock -forming minerals in thin sections . Enke Verlag 1987.
- PH Ribbe: Reviews in mineralogy Volume 5: Orthosilicates . Mineralogical Society of America, Washington, 2nd Edition, 1982
- JV Smith and WL Brown: Feldspar Minerals, Volume 1 . Springer-Verlag, Berlin, 2nd edition; 1988
- H. Strunz: Strunz mineralogical tables: chemical structural mineral classification system. 9th edition, Schweizerbart, Stuttgart. ISBN 978-3-510-65188-7
- WE Tröger: Optical determination of rock-forming minerals, Part 1: Determination tables . Schweizerbart'sche Verlagsbuchhandlung, Stuttgart, 4th edition, 2001.
- WE Tröger: Optical determination of rock-forming minerals, Part 2: Text volume . Schweizerbart'sche Verlagbuchhandlung, Stuttgart,?. Edition, 1967.
- T. Zoltai: Classification of silicates and other minerals with tetrahedral structures. In: American mineralogist . 45, 960-973, 1960
Web links
- Mineral Atlas: Silicate (Wiki)
- Complete lecture on silicates from ruby.chemie.uni-freiburg.de
- Mineralogie-web.de
Individual evidence
- ↑ WeylClean® SKS-6 - The WeylChem Group. Accessed August 9, 2019 .
- ↑ Werner Stöber, Arthur Fink, Ernst Bohn: Controlled growth of monodisperse silica spheres in the micron size range. In: Journal of Colloid and Interface Science. 26, 1968, pp. 62-69, doi : 10.1016 / 0021-9797 (68) 90272-5 .
- ↑ Christian Schittich, Gerald Staib , Dieter Balkow, Matthias Schuler, Werner Sobek : Glass Construction Manual . Walter de Gruyter, 2006, ISBN 3-0346-1553-1 , p. 62 ( limited preview in Google Book search).



![{\ displaystyle \ mathrm {M_n \ left [\ left (Si_xO_y \ right) ^ {4x-2y} \ right]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5c87a4e9317d60e092bbfa12fe016989317f4eab)
![{\ displaystyle \ mathrm {Al_2 \ left [Si_2O_5 | (OH) _4 \ right]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/becfd5c3837eab509f18e757afad1e1b76a9412d)
![{\ displaystyle \ mathrm {Na [AlSi_2O_6] \ cdot H_2O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/49ce7b36050eb923fba911f9a5f09324b83f0ec3)
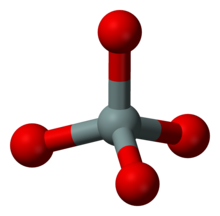
![{\ displaystyle \ mathrm {(Mg, Fe) _2 [SiO_4]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0f6d176c33cf50d1c311fa6fbe06e2dc17d7143d)
![{\ displaystyle \ mathrm {Zr [SiO_4]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e76a30cfbd1a061aa1ea321cda5fcb39bd09e348)