Sillimanite
| Sillimanite | |
|---|---|
| Sillimanite from Orissa, India | |
| General and classification | |
| chemical formula | Al 2 [O | SiO 4 ] |
|
Mineral class (and possibly department) |
Island silicates (nesosilicates) with additional anions; Cations in [4], [5] and / or only [6] coordination |
|
System no. to Strunz and to Dana |
9.AF.05 ( 8th edition : VIII / B.02) 52.02.02a.01 |
| Similar minerals | Andalusite , kyanite |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | orthorhombic-dipyramidal 2 / m 2 / m 2 / m |
| Space group | Pnma |
| Lattice parameters | a = 7.484 Å ; b = 7.672 Å; c = 5.77 Å |
| Formula units | Z = 4 |
| Frequent crystal faces | {010}, {110} |
| Twinning | no |
| Physical Properties | |
| Mohs hardness | 6.5 to 7.5 |
| Density (g / cm 3 ) | 3.24 |
| Cleavage | completely after {010} |
| Break ; Tenacity | uneven, brittle |
| colour | colorless, white, yellowish-gray, gray-green, light brown |
| Line color | White |
| transparency | transparent to translucent |
| shine | Glass shine, silky |
| Crystal optics | |
| Refractive indices |
n α = 1.653 to 1.661 n β = 1.654 to 1.670 n γ = 1.669 to 1.684 |
| Birefringence | δ = 0.016 to 0.023 |
| Optical character | biaxial positive |
| Axis angle | 2V = 21 to 30 ° |
| Pleochroism | weak (mostly colorless); otherwise X: pale brown or yellowish Y: brown or gray-green Z: dark brown or blue |
| Other properties | |
| Chemical behavior | not decomposable by HF |
| Special features | not grainy or coarse; Grown subparallel in quartz: fiber pebbles |
The mineral sillimanite is a very common island silicate from the group of aluminosilicates and has the chemical composition Al 2 SiO 5 or Al 2 [O | SiO 4 ]. It crystallizes in the orthorhombic crystal system and forms prismatic to fibrous crystals of small size. It is not uncommon to find a small amount of Fe 2 O 3 in sillimanite .
Sillimanite has a high hardness of 6.5 to 7.5 and a white-gray to green-gray color, but is sometimes colorless. The line color is white. Similar minerals with the same or similar chemical composition are andalusite , kyanite and mullite , which also belong to the aluminosilicates.
Etymology and history
Sillimanit was named after the American chemist Benjamin Silliman . A sillimanite mineral found in Chester , Connecticut , was first scientifically described by George T. Bowen in 1824. Sillimanite is sometimes also referred to as Bucholzit - after the German pharmacist and chemist WHS Bucholz. It is also known as gloss spar .
classification
In the old (8th edition) and new systematics of minerals (9th edition) according to Strunz , sillimanite belongs to the division of "island silicates with non-tetrahedral anions (Neso-subsilicates)". The new Strunz'sche mineral system, however, subdivides here more precisely according to the position of the cations in the crystal, so that the mineral now belongs to the subdivision of "island silicates with additional anions and cations in [4] -, [5] - and / or only [6]" -Coordination ", where he is the only member of the unnamed group 9.AF.05 .
The systematics of minerals according to Dana , which is common in the English-speaking world , also assigns sillimanite to the class of silicates, but there in the division of “ island silicates with SiO 4 groups and O, OH, F and H 2 O with cations in [4] and > [4] coordination ", where he served as eponymous mineral, together with mullite the" Al2SiO5 (sillimanite subgroup) "with the system no. 52.2.2a forms.
Crystal structure
Sillimanite crystallizes in the orthorhombic-dipyramidal crystal system in the space group P nma with the lattice parameters a = 7.484 Å ; b = 7.672 Å and c = 5.77 Å as well as four formula units per unit cell .
The basic building blocks of the sillimanite structure are:
- Four- coordinate [SiO 4 ] and [AlO 4 ] tetrahedra and
- [AlO 6 ] octahedra in six-coordination.
Aluminum thus plays a dual role, i. H. in two different coordination levels, as Al [4] and as Al [6] , a more precise formula for sillimanite is therefore also: Al [6] [O | Al [4] Si [4] O 4 ].
The octahedra are linked by their parallel side edges, lined up in endless chains and run parallel to the c-axis. One strand lies in the center of the unit cell, four more form the side edges parallel to the c-axis. The tetrahedra also form four endless chains in the c-direction, with the central atoms Si and Al regularly alternating with one another. These Si-Al-Si-Al ....- tetrahedron chains lie between the octahedral chains and are linked to the octahedral chains via their oxygen atoms (O D atoms). The tetrahedron chains , however, are not single single chains but single double chains ; This means that they are linked to the opposite tetrahedron chain of the neighboring unit cell via their free tips (O C atoms).
Due to this arrangement, sillimanite can also be regarded as a chain silicate (inosilicate); this also explains its elongated, needle-like, fibrous habit very well.
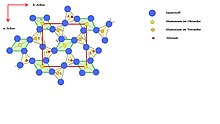
To illustrate the sillimanite structure opposite figure:
The projection of half a unit cell (from Z = 0 to Z = 1/2) along the c-axis onto the ab or (001) plane is shown. The unit cell is outlined in red. The [AlO 6 ] octahedra are light green, those of the [SiO 4 ] and [AlO 4 ] tetrahedra are highlighted in beige. This form of representation was chosen for reasons of clarity, since only the central atoms of the octahedra (Al 1 atoms) and the O D atoms strictly maintain their parallelism to the c-axis; all other atoms are slightly shifted in their position in the upper half of the unit cell.
The figure on the left is a simplified elevation of the unit cell parallel to the c-axis. It shows very nicely linking the tetrahedral One double chains with Oktaederkette and the parallelism of the atomic positions Al 1 and O D . Noteworthy is the dimensional difference between the silicon tetrahedron and the aluminum tetrahedron (2.696 and 3.074 Å), which add up to the dimension of the c-axis in the unit cell (5.77 Å).
The unit cell of Sillimanit
| Atomic position | a-axis | b-axis | c-axis |
|---|---|---|---|
| Al 1 | 0.0000 | 0.0000 | 0.0000 |
| Al 2 | 0.1418 | 0.3449 | 0.2500 |
| Si | 0.1535 | 0.3402 | 0.7500 |
| O A | 0.3600 | 0.4088 | 0.7500 |
| O B | 0.3563 | 0.4340 | 0.2500 |
| O C | 0.4765 | 0.0017 | 0.7500 |
| O D | 0.1256 | 0.2232 | 0.5144 |
The atomic positions of the unit cell of sillimanite are as follows:
This information and 13 subsequent symmetry operations are sufficient to fully define the unit cell.
The almost identical dimensions of the a- and b-axis are remarkable, sillimanite therefore only slightly misses tetragonal symmetry.
X-ray diffractometry
| Intensity (I / I 0 ) | Grid spacing (d) in Å | Angle (2-theta) | Area (hkl) |
|---|---|---|---|
| 100 (also 65) | 3.365 | 26.48 ° | (210) |
| 79.65 (also 100) | 3.417 | 26.08 ° | (120) |
| 67.37 | 2.206 | 40.91 ° | (122) |
| 49.66 | 2.543 | 35.29 ° | (112) |
| 41.88 | 1.519 | 60.96 ° | (332) |
X-ray diffraction studies on sillimanite crystals have provided the following results:
The first two maximum values are very close together and are often interchanged. The diffraction angle (2-theta) is given for Cu K-alpha radiation.
properties
In the petrology of metamorphic rocks, sillimanite plays an important role as an indicator for the strength of the transformations. The so-called index mineral defines its first occurrence as the sillimanite zone or the sillimanite isograde , its stability range being limited by the thermodynamic transformations andalusite ⇔ sillimanite and kyanite ⇔ sillimanite. This area is at relatively high temperatures (> 540 ° C) and can reach medium pressures (up to ~ 1 GPa, corresponding to a depth of 36.5 kilometers). It mostly spans the amphibolite and granulite facies as well as the high-temperature contact metamorphosis .
In the course of metamorphosis, new sillimanite is formed by polymorphic transformation from andalusite or kyanite or by conversion reactions of biotite and muscovite. The following reaction is given as an example:
- 1 muscovite + 1 quartz ⇒ 1 sillimanite + 1 alkali feldspar + 1 water
- 1 KAl 2 [(OH) 2 | AlSi 3 O 10 ] + 1 SiO 2 ⇒ 1 Al 2 SiO 5 + 1 KAlSi 3 O 8 + 1 H 2 O
This reaction is very important because it divides the stability field of sillimanite into two areas - the sillimanite zone is therefore also subdivided into two sub-zones , the slightly lower temperature and pressure-stressed sillimanite muscovite subzone and the higher temperature sillimanite alkali feldspar subzone . The reaction begins to take effect from 630 ° C and causes the complete disappearance of muscovite.
Reactions between staurolite and biotite or between staurolite and quartz.
When anatectic temperatures are reached and exceeded, reactions occur in which sillimanite is broken down again. As examples the biotite dehydrations:
Sillimanite + biotite ⇒ garnet + alkali feldspar + liquid or
Sillimanite + biotite ⇒ garnet + cordierite ± liquid
But even in the course of the retromorphism , sillimanite gradually disappears again, with falling temperatures and pressure drop z. B. andalusite regressed polymorphically.
Sillimanite is a fairly weather-resistant mineral, but it still decomposes with the formation of kaolinite and muskowite or sericite (epizonal sericite ).
Modifications and varieties
Sillimanite is the high-temperature, low-pressure modification of the Al 2 SiO 5 group and is trimorphic with the other members andalusite and kyanite .
Fibrolite is a tufted aggregate of elongated sillimanite crystals (Comte de Bournon, 1802). Fiber pebbles , on the other hand, are sub-parallel, needle-like swarms and strands of sillimanite in quartz or cordierite (described by Lindacker in Bohemia in 1792). Other local varieties are Monrolit (after the city of Monroe in New York State ) and Bamlit (after Bamle near Brevik in Norway ).
Education and Locations
Sillimanite can be found in the form of stem-like, fibrous or columnar crystals or massive in aluminum-rich , pelitic, regionally metamorphic rocks . It usually occurs in two types of metamorphosis:
- In the Abukuma type at relatively low pressures in mica schists.
The accompanying mineral is mostly andalusite . - In the Barrow type at medium pressures in gneiss.
Accompanying minerals are kyanite and cordierite .
Sillimanite occurs in contact metamorphic form in the highest temperature sanidinite facies.
As a mineral of magmatic origin, it is part of peraluminous granitoids. Sillimanite is rarely found in amphibolites and eclogites , relatively rarely in pegmatites , but quite often in granulites . It is also occasionally found as detritus in sediments.
Accompanying minerals are alkali feldspar , almandine , andalusite , biotite , cordierite , enstatite (at higher temperatures) corundum , kyanite , muscovite , plagioclase , quartz and / or spinel .
The type locality for Sillimanit is Sušice in the Czech Republic . Sites in Germany are the Laacher See , the Spessart and Bodenmais in the Bavarian Forest . Worldwide: Sellrain (Austria), Auvergne (France), Meghalaya ( Northeast India ), Myanmar , Sri Lanka , Enderbyland ( Antarctica ) and Brandywine Springs ( Delaware , USA).
use
Sillimanite is used as a gemstone if it is of good quality , but little is known so far. Clear varieties are usually offered in different facet cuts such as brilliant or faceted oval cut . Opaque stones and those with optical effects such as chatoyance ( cat's eye effect ) or asterism ( star effect ), on the other hand, are given a cabochon-shaped smooth cut.
Sillimanit is used industrially to manufacture refractory materials (support tubes for heating coils in electrical furnace construction, spark plugs, etc.).
See also
Individual evidence
- ↑ a b c d Webmineral - Sillimanite (engl.)
- ↑ a b c Sillimanite at mindat.org (engl.)
- ↑ WE Tröger: Optical determination of the rock-forming minerals .4. revised edition. E. Schweizerbart'sche Verlagbuchhandlung, 1971, ISBN 3-510-65011-5 (p. 51)
- ↑ a b Burnham, CW (1963a). Refinement of the crystal structure of sillimanite. Z. Kristallogr., 118, 127-148
- ↑ Peterson, RC & McMullan, RK (1986). Neutron diffraction studies of sillimanite. At the. Min., Vol 71, p742-745
- ^ Database-of-Raman-spectroscopy - Sillimanite
- ↑ Spear, FS, Kohn, MJ, and Cheney, JT, 1999, PT paths from anatectic pelites: Contributions to Mineralogy and Petrology, v. 134, p. 17-32, doi: 10.1007 / s004100050466 . Contains data on the position of the aluminosilicate triple point.
- ↑ Wildlife Institute of India: Table 2.2: Minerals of Meghalaya and Fig 2.3: Mineral Map of Meghalaya In: The Meghalaya State Biodiversity Strategy and Action Plan (2016-2026; Draft). Ministry of Environment Forest and Climate change, Government of India 2017 (English, without page numbers; here PDF pages 28/29; full text: PDF: 15.4 MB, 350 pages at megbiodiversity.nic.in); Quote: "The Sonapahar sillimanite area of West Khasi Hills District is the only area in the state [Meghalaya] where lensoid bodies of massive sillimanite mineral are found. Total reserve of 55 MTs (GSI, 2009), which is about 95% of India's total reserve. "
- ↑ realgems.org - sillimanite (with depictions of various rough and faceted stones)
literature
- Petr Korbel, Milan Novák: Encyclopedia of Minerals . Nebel Verlag GmbH, Eggolsheim 2002, ISBN 3-89555-076-0 , p. 201 .
- Martin Okrusch, Siegfried Matthes: Mineralogy: An introduction to special mineralogy, petrology and deposit science . 7th edition. Springer Verlag, Berlin, Heidelberg, New York 2005, ISBN 3-540-23812-3 , pp. 84 .
- Walter Schumann: Precious stones and gemstones. All species and varieties in the world. 1600 unique pieces . 13th revised and expanded edition. BLV Verlags GmbH, Munich a. a. 2002, ISBN 3-405-16332-3 , pp. 234 .
Web links
- Mineral Atlas: Sillimanite (Wiki)