Garnet group
| garnet | |
|---|---|
| Almandine from the Garnet Ledge near Wrangell , Wrangell Island , Alaska with rhombic dodecahedron and trapezoidal surfaces (size 2.3 cm × 2.3 cm × 2.2 cm) | |
| General and classification | |
| chemical formula | general structural formula: [8] X 3 [6] Y 2 ( [4] ZO 4 ) 3 |
|
Mineral class (and possibly department) |
Silicates and germanates - island silicates (nesosilicates) |
|
System no. to Strunz and to Dana |
9.AD.25 ( 8th edition : VIII / A.08) April 51, 03a to April 51, 2003 and April 51, 2004 |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic hexakisoctahedral; 4 / m 3 2 / m |
| Frequent crystal faces | {110}, {211} |
| Physical Properties | |
| Mohs hardness | 6.5 to 7.5 |
| Density (g / cm 3 ) | 3.5 to 4.3 |
| Cleavage | imperfect |
| Break ; Tenacity | shell-like, splintery, brittle |
| colour | variable, often red-brown, yellow-green, black |
| Line color | White |
| transparency | transparent to opaque |
| shine | Glass, fat, resin gloss |
| magnetism | ferrimagnetic |
The garnet group (short grenade ) is an important group of rock-forming minerals from the department of island silicates (nesosilicates).
The general garnet formula is: [8] X 3 [6] Y 2 [ [4] ZO 4 ] 3 or A 3 2+ B 2 3+ [RO 4 ] 3 , where 'X', 'Y' and 'Z 'or' A ',' B 'and' R 'do not represent chemical elements , but represent defined places in the crystal lattice. The respective grid positions can be occupied by different ions :
- X or A: predominantly divalent cations , dodecahedral surrounded by eight oxygen anions, mostly Mg 2+ , Fe 2+ , Mn 2+ and Ca 2+ but also Y 3+ or Na +
- Y or B: predominantly trivalent cations, octahedral surrounded by six oxygen anions, mostly Al 3+ , Fe 3+ , Cr 3+ and V 3+ , but also Ti 4+ , Zr 4+ , Sn 4+ , Sb 5+ or Mg 2+ , Mn 2+
- Z or R: predominantly tetravalent cations surrounded by four oxygen anions, mostly Si 4+ , but also Al 3+ , Fe 3+ , Ti 4+ , P 5+ , As 5+ , V 5+
- Anion: mostly O 2- , rarely also (OH) - or F -
Within the garnet top group, which includes all minerals that crystallize with the garnet structure, including those from other mineral classes (e.g. halides, hydroxides), the garnet group includes all minerals with 12 positive charges per formula unit in the Z position. Currently (2013) these are only silicates .
The garnet minerals mostly crystallize in the cubic crystal system and form predominantly isometric crystals with the characteristic shapes of the rhombic dodecahedron (outdated also garnethedron ), icositetrahedron and their combinations.
Garnets are generally transparent to translucent, with many foreign admixtures and in coarse mineral aggregates also opaque. Undamaged or unweathered crystal surfaces have a glass-like to grease-like gloss . The color of the garnet is very variable, even if reddish color varieties predominate. The palette ranges from light green to yellow-green to dark green, light yellow to yellow-orange and orange-red as well as from a light pink to an almost black-looking dark red. Colorless and brown varieties are less common, and very rarely also color-changing ( shimmering ) and blue garnets. However, the line color is always white.
Their relatively high density (3.5 to 4.5 g / cm 3 ), Mohs hardness (6.5 to 7.5) and light refraction (n = 1.61 ( katoite ) to n = 1.96 ( calderite )) interesting both as a gemstone and for industrial applications.
Etymology and history
The term garnet was first coined in the Middle Ages , but has its origins in the Latin word granum for grain or kernel or granatus for grainy or kernel-rich and on the one hand refers to the occurrence of the mineral in grains, which is similar to the pomegranate kernels ( Punica granatum ), on the other hand also on the orange-red to red-violet color of the flower, fruit and seeds of the pomegranate.
Garnets were already used as gemstones in ancient times . In the Middle Ages they were known together with rubies and spinels under the designation carbuncle (also carbuncle stone ) - most of them came from India at that time. But they were particularly popular in the 19th century, when Bohemian pyropes were so popular that they were shipped to America.
classification
Strunz
In the now outdated, but still in use 8th edition of the mineral classification according to Strunz , the garnet group belonged to the general department of " island silicates (nesosilicates)", bearing the system no. VIII / A.08 and consisted of the members almandine , Andradite , Calderit , Goldmanit , Grossular , Henritermierit , Hibschite , Holtstamit , Hydrougrandit (discredited 1967 as unnecessary group name), katoite , Kimzeyit , Knorringit , majorite , Morimotoit , pyrope , Schorlomit , Spessartin , Uwarowit , Wadalit and Yamatoit (discredited because they are identical to Momoiit).
The 9th edition of Strunz's mineral classification, which has been in effect since 2001, also assigns the garnet group to the “island silicates” department. However, this is further subdivided according to the possible presence of further anions and the coordination of the cations involved , so that the garnet group with the system no. 9.AD.25 according to the composition of the members almandine, andradite, blythite , calderite, goldmanite, grossular, henritermierite , hibschite, holtstamite , hydroandradite , katoite , kimzeyite , knorringite , majorite , momoiite ( IMA 2009-026 ), morimotoite , pyrope , Schorlomit , Spessartin, Skiagit , Uwarowit and Wadalit in the subsection “Island silicates without further anions; Cations in octahedral [6] and usually greater coordination ”can be found.
Dana
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the garnet group to the section of "island silicate minerals ". Here, however, it is divided into the subgroups “Pyralspit series” (system no. 51.04.03a ), “Ugrandite series” (system no. 51.04.03b ), “Schorlomit-Kimzeyit series” (system no. 51.04.03c ), " Hydro grenades " (system no. 51.04.03d ) and "Tetragonal hydro grenades " (system no. 51.04.04 ) within the subsection " Island silicates: SiO 4 groups only with cations in [6] and> [6] coordination ”.
IMA / CNMNC
The classic classifications listed above determine the main groups (classes) based on their composition and subdivide them according to structural criteria.
The current classification of the grenade, which was developed by the International Mineralogical Association (IMA) in 2013, works in reverse. It defines the garnet upper group on the basis of the structure type and divides it according to chemical aspects, the cation charge on the tetrahedrally coordinated Z position, into 5 groups and 3 individual minerals. In addition, a classification according to the charge distribution on the grid positions was inserted and hypothetical end links added.
| Garnet upper group: minerals with a garnet structure | ||||||
|---|---|---|---|---|---|---|
| Surname | class | Kenogranat group: unoccupied Z-position | annotation | |||
| [8] M 2+ 3 | [6] M 3+ 2 | [4] □ 3 | X - 12 | |||
| Katoite | Hydroxides | Approx 3 | Al 2 | □ 3 | (OH) 12 | |
| Hydroxides | Approx 3 | Fe 3+ 2 | □ 3 | (OH) 12 | hypothetical end link, up to 35 mol% in andradite, synthetic | |
| Halide | Approx 3 | Al 2 | □ 3 | F 12 | hypothetical end link, up to 11 mol% in hydrogrossular | |
| Halide | Mn 2+ 3 | Al 2 | □ 3 | F 12 | hypothetical terminal, up to 8 mol% in Spessartine | |
| Surname | class | unnamed group: halides with 3 charges on Z | annotation | |||
| [8] M + 3 | [6] M 3+ 2 | [4] M + 3 | X - 12 | |||
| Cryolithionite | Halides | Well 3 | Al 2 | Li 3 | F 12 | |
| Surname | class | unnamed group: oxides with 6 charges on Z | annotation | |||
| [8] M 2+ 3 | [6] M 6+ 2 | [4] M 2+ 3 | X 2- 12 | |||
| Yafsoanite | Oxides | Approx 3 | Te 6+ 2 | Zn 3 | O 12 | |
| Oxides | Pb 3 | Te 6+ 2 | Zn 3 | O 12 | hypothetical end link, up to 9 mol% in yafsoanite | |
| Oxides | Approx 3 | U 6+ 2 | Fe 2+ 3 | O 12 | hypothetical end link, up to 24 mol% in elbrusite | |
| Surname | class | Henritermierite group: Silicates with 8 charges on Z | annotation | |||
| [8] M 2+ 3 | [6] M 3+ 2 | [4] (M 4+ 2 □) | X 2- 8 X - 4 | |||
| Holtstamit | Silicates | Approx 3 | Al 2 | Si 2 □ | O 8 (OH) 4 | |
| Henritermierite | Silicates | Approx 3 | Mn 3+ 2 | Si 2 □ | O 8 (OH) 4 | |
| Silicates | Mn 2+ 3 | Al 2 | Si 2 □ | O 8 (OH) 4 | hypothetical terminal, up to 28 mol% in Spessartine | |
| Silicates | Mn 2+ 3 | Al 2 | Si 2 □ | O 8 F 4 | hypothetical terminal, up to 20 mol% in Spessartine | |
| [8] M 2+ 3 | [6] M 5+ 2 | [4] (M 3+ 2 M 2+ ) | X 2- 12 | |||
| Montenegro | Oxides | Approx 3 | Sb 5+ 2 | Fe 3+ 2 Fe 2+ | O 12 | |
| Oxides | Approx 3 | Sb 5+ 2 | Fe 3+ 2 Zn 2+ | O 12 | hypothetical end member, 20 mol% in Montenegro | |
| Surname | class | Bititicleit group: oxides with 9 charges on Z | annotation | |||
| [8] M + 3 | [6] M 6+ 2 | [4] M 3+ 3 | X 2- 12 | |||
| Oxides | Well 3 | Te 6+ 2 | Fe 3+ 3 | O 12 | synthetic | |
| [8] M + M 2+ 2 | [6] M 5+ 2 | [4] M 3+ 3 | X 2- 12 | |||
| Oxides | NaCa 2 | Sb 5+ 2 | Fe 3+ 3 | O 12 | synthetic | |
| [8] M 2+ 3 | [6] (M 5+ 1.5 M 3+ 0.5 ) | [4] M 3+ 3 | X 2- 12 | |||
| Oxides | Approx 3 | Sb 5+ 1.5 Fe 3+ 0.5 | Fe 3+ 3 | O 12 | hypothetical end link, 33 mol% in Montenegro | |
| [8] M 2+ 3 | [6] (M 5+ M 4+ ) | [4] M 3+ 3 | X 2- 12 | |||
| Bitikleit | Oxides | Approx 3 | Sb 5+ Sn 4+ | Al 3 | O 12 | |
| Dzhuluit | Oxides | Approx 3 | Sb 5+ Sn 4+ | Fe 3+ 3 | O 12 | |
| Usturit | Oxides | Approx 3 | Sb 5+ Zr 4+ | Fe 3+ 3 | O 12 | |
| Elbrusite | Oxides | Approx 3 | U 5+ Zr 4+ | Fe 3+ 3 | O 12 | |
| [8] (M 4+ 0.5 M 2+ 2.5 ) | [6] M 4+ 2 | [4] M 3+ 3 | X 2- 12 | |||
| Oxides | Th 4+ 0.5 Ca 2+ 2.5 | M 4+ 2 | M 3+ 3 | O 12 | hypothetical terminal, up to 20 mol% in kerimasite | |
| [8] M 3+ 3 | [6] M 3+ 2 | [4] M 3+ 3 | X 2- 12 | |||
| YAG | Oxides | Y 3+ 3 | Al 3+ 2 | Al 3+ 3 | O 12 | hypothetical end link, up to 8 mol% in menzerite- (Y), spessartine, andradite |
| YIG | Oxides | Y 3+ 3 | Fe 3+ 2 | Fe 3+ 3 | O 12 | hypothetical end link, up to 8 mol% in menzerite- (Y), spessartine, andradite |
| Surname | class | Schorlomite group: Silicates with 10 charges on Z | annotation | |||
| [8] M 2+ 3 | [6] M 4+ 2 | [4] (M 4+ M 3+ 2 ) | X 2- 12 | |||
| Kimzeyit | Silicates | Approx 3 | Zr 2 | SiAl 2 | O 12 | |
| Irinarassit | Silicates | Approx 3 | Sn 4+ 2 | SiAl 2 | O 12 | |
| Hutcheonite | Silicates | Approx 3 | Ti 2 | SiAl 2 | O 12 | |
| Schorlomit | Silicates | Approx 3 | Ti 2 | SiFe 3+ 2 | O 12 | |
| Kerimasite | Silicates | Approx 3 | Zr 2 | SiFe 3+ 2 | O 12 | |
| Toturite | Silicates | Approx 3 | Sn 4+ 2 | SiFe 3+ 2 | O 12 | |
| Surname | class | Garnet group: Silicates with 12 charges on Z | annotation | |||
| [8] (M 3+ 2 M 2+ ) | [6] M 2+ 2 | [4] M 4+ 3 | X 2- 12 | |||
| Menzerite- (Y) | Silicates | Y 2 approx | Mg 2 | Si 3 | O 12 | |
| Silicates | Y 2 approx | Fe 2+ 2 | Si 3 | O 12 | hypothetical end link, up to 20 mol% in menzerite (Y) | |
| [8] M 2+ 3 | [6] M 3+ 2 | [4] M 4+ 3 | X 2- 12 | |||
| Pyrope | Silicates | Mg 3 | Al 2 | Si 3 | O 12 | |
| Grossular | Silicates | Approx 3 | Al 2 | Si 3 | O 12 | |
| Spessartine | Silicates | Mn 2+ 3 | Al 2 | Si 3 | O 12 | |
| Almandine | Silicates | Fe 2+ 3 | Al 2 | Si 3 | O 12 | |
| Eringait | Silicates | Approx 3 | Sc 2 | Si 3 | O 12 | |
| Goldmanite | Silicates | Approx 3 | V 3+ 2 | Si 3 | O 12 | |
| Rubinite | Silicates | Approx 3 | Ti 3+ 2 | Si 3 | O 12 | |
| Momoiit | Silicates | Mn 2+ 3 | V 3+ 2 | Si 3 | O 12 | |
| Knorringite | Silicates | Mg 3 | Cr 3+ 2 | Si 3 | O 12 | |
| Uvarowit | Silicates | Approx 3 | Cr 3+ 2 | Si 3 | O 12 | |
| Andradite | Silicates | Approx 3 | Fe 3+ 2 | Si 3 | O 12 | |
| Calderite | Silicates | Mn 2+ 3 | Fe 3+ 2 | Si 3 | O 12 | |
| Blythite | Silicates | Mn 2+ 3 | Mn 3+ 2 | Si 3 | O 12 | hypothetical end link |
| Khoharit | Silicates | Mg 2+ 3 | Fe 3+ 2 | Si 3 | O 12 | hypothetical end link, synthetic |
| Ski trip | Silicates | Fe 2+ 3 | Fe 3+ 2 | Si 3 | O 12 | hypothetical end link, synthetic |
| [8] M 2+ 3 | [6] (M 4+ M 2+ ) | [4] M 4+ 3 | X 2- 12 | |||
| Majority | Silicates | Mg 3 | SiMg | Si 3 | O 12 | |
| Morimotoite | Silicates | Approx 3 | TiFe 2+ | Si 3 | O 12 | |
| [8] (M 3+ 1.5 M + 1.5 ) | [6] M 3+ 2 | [4] M 4+ 3 | X 2- 12 | |||
| Silicates | (Y, Yb) 3+ 1.5 Na + 1.5 | M 3+ 2 | Si 3 | O 12 | hypothetical terminal, up to 7 mol% in almandine, spessartine, grossular | |
| [8] (M 2+ M + 2 ) | [6] M 4+ 2 | [4] M 4+ 3 | X 2- 12 | |||
| Silicates | M 2+ Na 2 | Si 2 | Si 3 | O 12 | hypothetical terminal, up to 12 mol% in pyrope grossular | |
| Surname | class | Berzeliite group: Vanadate / Arsenate with 15 charges on Z | annotation | |||
| [8] (M 2+ 2 M + ) | [6] M 2+ 2 | [4] M 5+ 3 | X 2- 12 | |||
| Shepherdite | Vanadates | Ca 2 Na | Mg 2 | V 5+ 3 | O 12 | |
| Palenzonaite | Vanadates | Ca 2 Na | Mn 2+ 2 | V 5+ 3 | O 12 | |
| Berzeliite | Arsenates | Ca 2 Na | Mg 2 | As 5+ 3 | O 12 | |
| Manganese berelite | Arsenates | Ca 2 Na | Mn 2+ 2 | As 5+ 3 | O 12 | |
| Arsenates | Ca 2 Na | Fe 2+ 2 | As 5+ 3 | O 12 | hypothetical terminal link, up to 6 mol% in berzeliite | |
| [8] M + 3 | [6] M 3+ 2 | [4] M 5+ 3 | X 2- 12 | |||
| Phosphates | Well 3 | Al 2 | P 5+ 3 | O 12 | hypothetical end member, up to 1 mol% in almandine and pyrope | |
Subgroups, obsolete names, and hypothetical end links
The IMA classification does not break down the grenade into subgroups. In older literature there is a division into two main groups / rows based on common mixed crystal rows:
Pyralspit group:
- Pyrope ( magnesium aluminum garnet ): Mg 3 Al 2 [SiO 4 ] 3
- Almandine ( iron-aluminum garnet ): Fe 3 Al 2 [SiO 4 ] 3
- Spessartine ( manganese aluminum garnet ): Mn 3 Al 2 [SiO 4 ] 3
Ugrandit group:
- Uwarowit ( calcium chromium garnet ): Ca 3 Cr 2 [SiO 4 ] 3
-
Grossular ( calcium aluminum garnet ): Ca 3 Al 2 [SiO 4 ] 3
- Grandit : Intermediate member of the mixed series Grossular-Andradit
- Plazolite : Intermediate link of the mixed series Grossular-Katoit
- Andradite ( calcium iron garnet ) Ca 3 Fe 3+ 2 [SiO 4 ] 3
Further names of mixed crystal compositions, hypothetical compositions or synthetic compounds:
- Hibschite : Ca 3 Al 2 [(SiO 4 ) > 1.5 ((OH) 4 ) <1.5 ], does not count as an independent mineral, but as an intermediate mixed crystal of the Grossular – Katoite series.
- Hydroandradite : Ca 3 Fe 3+ 2 [(SiO 4 ) > 1.5 ((OH) 4 ) <1.5 ], does not count as an independent mineral, but as a variety of andradite.
- Like chloromayenite , wadalite is no longer included in the upper garnet group. The deviations from the garnet structure are too great.
Modifications and varieties

- Akhtaragdite (also Akhtarandite , English Akhtaragdite ): Pseudomorphosis of grossular katoite mixed crystals (hydrogrossular) to mineral of the mayenite upper group , possibly also from Hibschit to Wadalit from Wiljui in Russia. Eightaragdite is usually found in the form of tetrahedral or triakiststraedic crystals from white-gray to gray-brown in color.
- Bredbergite (after James Dwight Dana , around 1900): Obsolete and no longer in use name for a magnesium-rich andradite variety
- Demantoid (after Nils von Nordenskiöld , around 1870): Andradite variety colored yellow-green by foreign admixtures
- Melanite (after Abraham Gottlob Werner , 1799): Is considered a titanium-rich variety of Andradite and was named after the Greek word μ Wortλας for black , as it occurs predominantly in gray-black to pitch-black crystals or coarse aggregates.
- Topazolith (for PC Bonvoisin, 1806): Light yellow, " topaz similar " Andradite variety, for the first time in Valle di Lanza in the Italian region of Piedmont was discovered
- Xalostocite : Name for a dense intergrowth of translucent pink-colored largeulars with white marble, which was named after the location Xalostoc in the Mexican state of Morelos .
Education and Locations
Grenades come in massive or granular form, but often also as macroscopic crystals that can weigh up to 700 kg.
Garnets are particularly common in metamorphic rocks such as gneiss , mica schist or eclogite ; they also occur in igneous rocks and sedimentary in heavy mineral soaps (beach sediments, river sediments). Most of the natural gemstone garnets found today come from the USA , South Africa and Sri Lanka .
The exact chemical composition is always related to that of the surrounding rock: For example, the magnesium-rich pyrope often occurs in peridotites and serpentinites , while green uvarovite occurs mainly in chromium-containing serpentinite rock.
Metapelite ( mica slate , gneiss )
During the metamorphosis of silicate pelites , garnets rich in almandine form from approx. 450 ° C in the reaction of chloritoid + biotite + H 2 O to garnet + chlorite . At low temperatures, the garnet mixed crystals are rich in spessartine and, as the temperatures rise, they increasingly contain almandine. From approx. 600 ° C, garnet forms when staurolite is broken down . As the temperatures continue to rise, the grenades become increasingly richer in pyrope and even when the rock begins to melt, grenades can still be formed anew. B. in the reaction of biotite + sillimanite + plagioclase + quartz to garnet + potassium feldspar + melt. Garnet only degrades into spinel + quartz at temperatures above 900 ° C or into orthopyroxene + sillimanite at high pressures .
Metabasite ( granulite , eclogite )
In the suite of metabasites (e.g. metamorphic basalts ), garnet occurs to form rock in eclogites, and the garnet mixed crystals are rich in pyrope and grossular .
With increasing pressure, garnet forms at the transition from granulite facies to eclogite facies from approx. 10 kbar, 900 ° C when orthopyroxene and plagioclase react to garnet, clinopyroxene and quartz . In blueschists initially Fe-rich shell that on the way to form eclogite facies increasingly Pyrop- and grossularite richer.
Decomposition of Garnet (Retrograde Conversions)

Under certain lithofacial circumstances, garnets undergo transformation or decomposition within metamorphic rocks. The result of these processes is called kelyphite . This creates numerous new minerals.
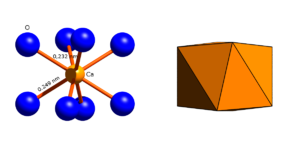
Crystal structure
Garnets generally crystallize with cubic symmetry in space group Ia 3 d (space group no. 230) . The unit cell contains 8 formula units and, depending on the composition, has an edge length of 1,146 nm (pyrope) to 1,256 nm (katoite).
Structure of the garnet structure
O 2− anion
The oxygen anions occupy the general lattice position 96h with the point symmetry 1 . Each O 2− anion is surrounded by 4 cations:
- a Z cation linked by a strong, predominantly covalent bond with approx. 1 bond valence
- a Y-cation to which there is a predominantly ionic bond with approx. 0.5 vu (valence units)
- two X cations, to which there are only weak, ionic bonds of approx. 0.25 vu each.
Unlike other high-density oxide structures, the oxygen ions do not form the closest packing of spheres . Large 8-fold coordinated ions would not find a place in a cubic or hexagonal close packing of oxygen spheres. Due to the complex connection of all coordination polyhedra via common corners and, above all, many common edges, the garnet structure nevertheless achieves a high density.
Depending on their size and charge, the cations occupy three different, special lattice positions where they are surrounded by 4, 6 or 8 oxygen ions.
ZO 4 tetrahedron
The Z cations (Si 4+ ) sit on the lattice position 24d with the point symmetry 4 , where they are surrounded by 4 oxygen ions which are located at the corners of a tetrahedron.
The ZO bond lengths determined are between 0.163 nm (pyrope, almandine) and 0.165 nm (goldmanite). The ZO 4 coordination tetrahedra have two pairs of edges of different lengths:
- two opposing edges that are not adjacent to any occupied lattice position with lengths between 0.274 nm (almandine, andradite) and 0.276 nm (goldmanite)
- two opposite sides, which border on XO 8 dodecahedra with lengths between 0.250 nm (pyrope, almandine) and 0.258 nm (uwarowite, goldmanite).
YO 6 octahedron
The Y cations sit on the lattice position 16a with the point symmetry 3 , where they are surrounded by 6 oxygen ions, which are located at the corners of an octahedron. The YO bond lengths determined are between 0.19 nm (pyrope) and 0.20 nm (andradite). The GO 6 coordination polyhedron has two different edges:
- 6 edges that do not border any occupied grid position with lengths between 0.262 nm (pyrope) and 0.289 nm (andradite)
- 6 edges that border on the X position with lengths between 0.269 nm (grossular) and 0.283 nm (andradite)
XO 8 dodecahedron
The X cations sit on the lattice position 24c with the point symmetry 222, where they are surrounded by 8 oxygen ions which are located at the corners of a dodecahedron ( trigondodecahedron ). The XO bond lengths determined are between 0.22 nm (pyrope) and 0.25 nm (andradite, glodmanite). The XO 8 coordination polyhedron has 4 different edges:
- 2 edges that border on neighboring ZO 4 tetrahedra with lengths between 0.250 nm (pyrope, almandine) and 0.258 nm (uwarowite, goldmanite).
- 4 edges that border on neighboring YO 6 octahedra with lengths between 0.269 nm (grossular) and 0.283 nm (andradite)
- 4 edges that border on adjacent XO 8 dodecahedra with lengths between 0.27 nm (pyrope) and 0.297 nm (grossular)
- 8 edges that do not border any occupied grid position with lengths between 0.278 nm (pyrope) and 0.287 nm (grossular, gold amnite)
Linking the coordination polyhedra
The ZO 4 tetrahedra and YO 6 octahedra are connected via shared oxygen atoms at their corners to form a framework of alternating tetrahedra and octahedra. Grenades are island silicates and their ZO 4 tetrahedra are not directly connected to one another.
The XO 8 dodecahedra are linked by common edges to form rings of three, the plane of which is perpendicular to the spatial diagonal of the unit cell. These XO 8 dodecahedron rings are linked to one another to form a framework that each dodecahedron belongs to two such rings. The dodecahedra are connected with the tetrahedra and octahedra of the ZO 4 -YO 6 framework, the spaces between which it fills.
Lowering of symmetry
Grossular andradite mixed crystals in particular are weakly birefringent and optically biaxial. Optical anisotropy was also observed with almandine. The optical properties are very sensitive indicators of deviations from the ideal, cubic structure. On the other hand, they could only rarely be detected in X-ray structure examinations of grenades. Some studies show triclinic (space group I 1 (No. 2, position 4) ) or ortorhombic (space group Fddd (no. 70) ), but also tetragonal (space group I 4 1 / acd (no. 142) ) or monoclinic for such grenades (Space group C 2 / m (No. 12) ) Symmetry. The causes of this lowering of symmetry are numerous:
- Plastic deformation
- Lowering of symmetry due to grid stresses
- Magneto-optical effects through the incorporation of rare earth elements
- Different distribution (order) of cations on different octahedral positions
- Lowering of symmetry through orderly incorporation of OH groups
use
As an abrasive
Because of its hardness, garnet is also used as an abrasive in sandblasting and water jet cutting.
In technology
In particular, artificially produced crystals with a garnet structure are used in precision mechanical and optical instruments. In contrast to the natural minerals, instead of silicon, other elements are often incorporated here on the tetrahedron square. Yttrium aluminum garnet (YAG, Y 3 Al 2 [Al O 4 ] 3 ), in which about one percent of the yttrium 3+ ions are replaced by neodymium 3+ ions, is a laser crystal ( Nd: YAG- Laser ), as well as Yb: YAG laser doped with ytterbium and Er: YAG laser doped with erbium . The yellow luminescence converter of the white LEDs was a cerium-doped YAG at the beginning of the development. Yttrium-iron-garnet (YIG) and relatives are used as microwave ferrite, resonators or as YIG filters in high-frequency technology.
In the 1970s and 1980s, grenades were used in the manufacture of magnetic bubble memory because of their special ferrimagnetic properties .
As a gem
Grenades are used in various forms as gemstones . One differentiates among other things the dark red pyrope, which is also called kaprubin, the red black almandine, the emerald green uwarowite, the yellow green andradite, the black schorlomite and melanite, the transparent-greenish demantoid and the orange-red Spessartin. There is also grossular. In addition, there has been a new variant for several years, the orange mandarin garnet. Grenades are also known as the little man's gems.
Polish imperial eagle (manufactured around 1638), consisting mainly of garnet (body) and ruby (wings, tail)
Uvarovite pendant , about 2 cm long
See also
literature
Monographs
- Thomas Fehr, Maximilian Glas, Joachim Zang: Garnet. The minerals of the garnet group: precious stones, jewelry and lasers (= extraLapis . No. 9 ). Weise, Munich 1995, ISBN 3-921656-35-4 .
In compendia
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th, revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 460-466 .
- Helmut Schrätze, Karl-Ludwig Weiner: Mineralogy. A textbook on a systematic basis . de Gruyter, Berlin / New York 1981, ISBN 3-11-006823-0 , p. 666-681 .
- Paul Ramdohr , Hugo Strunz : Klockmann's textbook of mineralogy . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 .
Scientific articles
- S. Geller: Crystal chemistry of the garnets. In: Journal of Crystallography. Volume 125, 1967, pp. 1-47. ( PDF file; 2.1 MB )
- GA Novak, GV Gibbs: The Crystal Chemistry of the Silicate Garnets. In: American Mineralogist. Vol. 56, 1971, pp. 791-825. ( PDF file; 2.2 MB )
- H. Hirai, H. Nakazawa: Visualizing low symmetry of a grandite garnet on precession photograph. In: American Mineralogist. Vol. 71, 1986, pp. 1210-1213. ( PDF file; 2.2 MB )
- D. Brown, RA Mason: An Occurrence of Sectored Birefringence in Almandine from the Gagnon Terrane, Labrador. In: Canadien Mineralogist. Vol. 32, 1994, pp. 105-110. ( PDF file; 835 kB )
- FM Allen, PR Buseck: XRD, FTIR, and TEM studies of optically anisotropic grossular garnets. In: American Mineralogist. Vol. 73, 1988, pp. 568-584. ( PDF file; 1.6 MB )
- DK Teertstra: INDEX-OF-REFRACTION AND UNIT-CELL CONSTRAINTS ON CATION VALENCE AND PATTERN OF ORDER IN GARNET-GROUP MINERALS. In: Canadian Mineralogist. Vol. 44, 2006, pp. 341-346. ( PDF file; 192 kB )
- KJ Kingma, JW Downs: Crystal-structure analysis of a birefringent andradite. In: American Mineralogist. Vol. 74, 1989, pp. 1307-1316. ( PDF file; 1.0 MB )
- DT Griffen, DM Hatch, WR Phillips, S. Kulaksis: Crystal chemistry and symmetry of a birefringent tetragonal pyralspite 75 -grandite 25 -garnet. In: American Mineralogist. Vol. 71, 1992, pp. 399-406. ( PDF file; 1.1 MB )
Web links
- Mineralienatlas: Garnet und Mineralienatlas: Mineralienportrait / Garnet (Wiki)
- Alena Křížová: Garnet . In: RDK Labor . Reallexikon zur Deutschen Kunstgeschichte, digital edition, 2014, detailed article on the history of the garnet in arts and crafts
- Mindat - Garnet (English)
- Alexandrite effect garnet on YouTube
Individual evidence
- ↑ a b c d e f g h i j Hugo Strunz , Ernest H. Nickel: Strunz Mineralogical Tables . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 540-542 .
- ↑ a b c d e Edward S. Grew, Andrew J. Locock, Stuart J. Mills, Irina O. Galuskina, Evgeny V. Galuskin, And Ulf Hålenius: IMA Report - Nomenclature of the garnet supergroup. In: American Mineralogist. Vol. 98, 2013, pp. 785-811. ( PDF file; 1.1 MB )
- ↑ Hans Jürgen Rösler : Textbook of Mineralogy . 4th, revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 460-466 .
- ^ Friedrich Klockmann : Klockmanns textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke , Stuttgart 1978, ISBN 3-432-82986-8 (first edition: 1891).
- ↑ Mineralienatlas: Mineralportrait Garnet - Color-changing and blue garnets
- ↑ a b DK Teertstra: INDEX-OF-REFRACTION AND UNIT-CELL CONSTRAINTS ON CATION VALENCE AND PATTERN OF ORDER IN GARNET-GROUP MINERALS. In: Canadian Mineralogist. Vol. 44, 2006, p. 342 ( PDF file; 192 kB )
- ↑ a b c Andreas Karlsson, Dan Holtstam, Luca Bindi, Paola Bonazzi, Matthias Konrad-Schmolke: Adding complexity to the garnet supergroup: monteneveite, Ca3Sb5 + 2 (Fe3 + 2Fe2 +) O12, a new mineral from the Montenevemine, Bolzano Province, Italy . In: European Journal of Mineralogy . tape 32 , 2020, p. 77-87 ( researchgate.net [PDF; 5.2 MB ; accessed on May 15, 2020]).
- ↑ a b AP Dodokin, S. Lyubutin, BV Mill, VP Peshkov: Mössbauer Effect In Antiferromagnetic Substances With Garnet Structures . In: Soviet Physics JETP . tape 36 , no. 3 , 1973, p. 526-531 ( jetp.ac.ru [PDF; 200 kB ; accessed on May 2, 2020]).
- ↑ Thomas Fehr, Maximilian Glas, Joachim Zang: Granat. The minerals of the garnet group: precious stones, jewelry and lasers (= ExtraLapis . No. 9 ). Weise, Munich 1995, ISBN 3-921656-35-4 , p. 19 .
- ↑ Thomas Fehr, Maximilian Glas, Joachim Zang: Granat. The minerals of the garnet group: precious stones, jewelry and lasers (= ExtraLapis . No. 9 ). Weise, Munich 1995, ISBN 3-921656-35-4 , p. 9 .
- ↑ Thomas Fehr, Maximilian Glas, Joachim Zang: Granat. The minerals of the garnet group: precious stones, jewelry and lasers (= ExtraLapis . No. 9 ). Weise, Munich 1995, ISBN 3-921656-35-4 , p. 13 .
- ↑ Thomas Fehr, Maximilian Glas, Joachim Zang: Granat. The minerals of the garnet group: precious stones, jewelry and lasers (= ExtraLapis . No. 9 ). Weise, Munich 1995, ISBN 3-921656-35-4 , p. 3 .
- ↑ Eugeny Galuskin, Irina Galuskina: Achtarandite - sponge hibschite pseudomorph after wadalite-like phase: internal morphology and mechanism of formation. In: New Yearbook for Mineralogy - Treatises. Volume 178, Issue 1 2003, pp. 63-74. ( PDF 32 kB ; brief description)
- ↑ Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . 2nd Edition. Ott Verlag, Thun 1979, ISBN 3-7225-6265-1 , p. 272 .
- ↑ Elements of mineralogy: adapted to the use of seminaries and private students , by John Lee Comstock in the Google book search
- ↑ Giambattista Brocchi, Carl August von Bloedau: J. Brocchi's mineralogical treatise on the valley of Fassa in Tyrol: provided with additions, a map of the valley of Fassa and a sheet of mountain sections. in Google Book Search
- ↑ Thomas Fehr, Maximilian Glas, Joachim Zang: Granat. The minerals of the garnet group: precious stones, jewelry and lasers (= ExtraLapis . No. 9 ). Weise, Munich 1995, ISBN 3-921656-35-4 , p. 17 .
- ↑ Metamorphism of pelites in FS Spear 1993: Metamorphic Phase Equilibria and Pressure-Temperature-Time Paths. Pp. 337-391.
- ↑ Gibbs 1971, p. 791.
- ^ A b c d S. Geller: Crystal chemistry of the garnets. In: Journal of Crystallography. Volume 125, 1967, p. 4. ( PDF file; 2.1 MB )
- ↑ a b c Novac & Gibbs 1971 - Tables 1, 9
- ↑ FM Allen, PR Buseck: XRD, FTIR, and TEM studies of optically anisotropic grossular garnets. In: American Mineralogist. Vol. 73, 1988, Fig. 3, p. 571. ( PDF file; 1.6 MB )
- ^ D. Brown, RA Mason: An Occurrence of Sectored Birefringence in Almandine from the Gagnon Terrane, Labrador. In: Canadien Mineralogist. Vol. 32, 1994, pp. 105-110. ( PDF file; 835 kB )
- ↑ The numbering of this axis position does not correspond to the order of the International Tables for Crystallography , because it is not listed there.
- ↑ FM Allen, PR Buseck: XRD, FTIR, and TEM studies of optically anisotropic grossular garnets. In: American Mineralogist. Vol. 73, 1988, p. 580. ( PDF file; 1.6 MB )
- ↑ Kingma & Downs 1989, p. 1307.
- ↑ a b Hirai & Nakazawa 1986
- ↑ Griffen u. a. 1992
- ↑ Andre Aubry, Yves Dusausoy, Alain Laffaille, Jean. Protas: Determination et etude de la structure cristalline de l'henritermierite, hydrogrenat de symmetretrie quadratique. In: Bulletin de la Societe frangaise de Mineralogie et de Cristallographie. Vol. 92, 1969, pp. 126-133.
- ↑ FM Allen, PR Buseck: XRD, FTIR, and TEM studies of optically anisotropic grossular garnets. In: American Mineralogist. Vol. 73, 1988, Fig. 3, pp. 568-569. ( PDF file; 1.6 MB )