Perovskite
| Perovskite | |
|---|---|
| Perovskite from Magnet Cove, Arkansas | |
| General and classification | |
| chemical formula | CaTiO 3 |
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.CC.30 ( 8th edition : IV / C.10) 03/04/03/01 |
| Similar minerals | Uhligit, Latrappit, Tausonit |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | orthorhombic-dipyramidal; 2 / m 2 / m 2 / m |
| Space group | Pbnm (No. 62, position 3) |
| Lattice parameters | a = 5.39 Å ; b = 5.45 Å; c = 7.65 Å |
| Formula units | Z = 4 |
| Frequent crystal faces | {100}, {010}, {001} |
| Twinning | Penetration twins according to [010], less often according to [121] |
| Physical Properties | |
| Mohs hardness | 5.5 |
| Density (g / cm 3 ) | 4.0 |
| Cleavage | indistinct after {001} |
| Break ; Tenacity | shell-like |
| colour | black, partly red-brown to yellow |
| Line color | gray to white |
| transparency | translucent to opaque (opaque) |
| shine | Diamond to metallic luster |
| Crystal optics | |
| Refractive indices |
n α = 2.300 n β = 2.340 n γ = 2.380 |
| Birefringence | δ = 0.080 |
| Optical character | biaxial positive |
| Axis angle | 2V = 88 to 90 ° |
| Pleochroism | not observed |
| Other properties | |
| Special features | piezoelectric |
Perovskite is a relatively common mineral from the mineral class of " oxides and hydroxides " with the chemical composition CaTiO 3 . From a chemical point of view, it is a calcium - titanium oxide or calcium titanate, i.e. a compound from the group of titanates .
The perovskite structure is an important structure type for technically important compounds such as ferroelectrics , but the term perovskite structure refers to a cubic crystal structure , which is not present in the eponymous perovskite. Because the ionic radius of the Ca 2+ cations in CaTiO 3 is too small , the crystal structure of the actual perovskite is distorted, causing it to crystallize in the lower- symmetry orthorhombic crystal system. The crystals of perovskite usually form metallic-looking cube-like shapes with a black to red-brown color.
Etymology and history
The first description of perovskite comes from the German mineralogist Gustav Rose (1798-1873) from the year 1839. He discovered the unknown mineral in the Druse a rock sample from Achmatowsk near Zlatoust (Urals), he of the upper Bergmeister treasurer of St. Petersburg had received. Rose described the crystal form, determined the hardness (5.5 on the Mohs hardness scale ) and the density of the mineral and carried out numerous chemical tests, which enabled him to determine the components calcium and titanium (IV) oxide without any doubt. He named the new mineral perovskite after the Russian politician and mineralogist Lev Alexejewitsch Perowski (1792-1856). Akhmatovsk is today the type locality of perovskite.
Since the founding of the International Mineralogical Association , perovskite has been the internationally recognized mineral name for the naturally occurring calcium titanium oxide CaTiO 3 .
classification
Already in the outdated, but still in use 8th edition of the mineral classification according to Strunz , the perovskite belonged to the mineral class of "oxides and hydroxides" and there to the department of "oxides with the molar ratio of metal: oxygen = 2: 3", where he named the "Perovskite series" with the system no. IV / C.10 and the other members Barioperowskit , Isolueshit , Latrappit , Loparit- (Ce) , Lueshit , Macedonit , Natroniobit and Tausonit formed.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), assigns perovskite to the expanded section of "Oxides with the molar ratio of metal: oxygen = 2: 3, 3: 5 and comparable" a. This is further subdivided according to the relative size of the cations involved , so that the mineral can be found according to its composition in the sub-section “With large and medium-sized cations”, where together with Lueshite the “Perovskite-Lueshite Group” with the system -No. 4.CC.30 and the other members Barioperowskit, Lakargiit , Latrappit and Natroniobit forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns perovskite to the class of "oxides and hydroxides" and there in the "oxides" category. Here he is also the namesake of the "perovskite group" with the system no. 04.03.03 and the other members latrappite, loparite (Ce), lueshite, tauonite, isolueshite, barioperovskite and lakargiite within the subdivision of " Simple oxides with a cation charge of 3+ (A 2 O 3 ) ".
Crystal structure
 Crystal structure of perovskite |
|
| Crystal system | orthorhombic |
| Room group (no.) | Pbnm (No. 62) |
| Lattice parameters |
a = 5.39 Å b = 5.45 Å c = 7.65 Å |
| Formula units | Z = 4 |
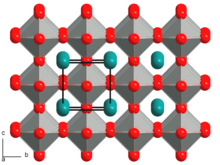
Perovskite crystallizes in the orthorhombic crystal system in the space group Pbnm (space group no. 62, position 3) with the lattice parameters a = 5.39 Å , b = 5.45 Å and c = 7.65 Å as well as four formula units per unit cell .
Ideally, perovskite would crystallize in the cubic crystal system in the space group Pm 3 m (space group no. 221) with a lattice parameter of the unit cell of approx. 3.80 Å. However, due to the too small ionic radius of the Ca 2+ cations, the crystal structure of CaTiO 3 is distorted, which explains the lower orthorhombic symmetry. The term perovskite structure as an important structure type usually refers to the cubic crystal structure in which the eponymous prototype itself does not crystallize.
The analogous compounds of the larger homologues of calcium, strontium and barium , on the other hand, crystallize in the undistorted cubic structure: Strontium titanate SrTiO 3 , also known as the mineral tauonite , with a lattice parameter of a = 3.90 Å; Barium titanate BaTiO 3 , also known as a mineral since 2007 ( barioperovskite ), with a = 4.01 Å and only one formula unit per unit cell. The degree of distortion of a compound ABO 3 in the perovskite structure can also be estimated using Goldschmidt's tolerance factor t , which is defined as follows:
where r A is the radius of the A cation, r B is the radius of the B cation, and r O is the radius of the anion (usually oxygen). The perovskite structure exists in the range 0.89 <t <1.02 , where t = 1 corresponds to the strontium titanate in the undistorted cubic structure.
The crystal structure of perovskite can be described in two different ways. The titanium atoms are each surrounded by six oxygen atoms in the shape of octahedra . These [TiO 6 ] octahedra form a three-dimensional network over common corners, which can be described using the Niggli notation as follows:
The gaps in this network contain the calcium atoms , which have a coordination sphere of twelve oxygen atoms in the form of a cuboctahedron as a coordination polyhedron . Alternatively, the structure can also be described as a cubic closest packing of spheres , which is built up jointly by calcium and oxygen. Every fourth octahedral gap in the spherical packing is occupied by titanium, namely the one that is only surrounded by oxygen atoms. Since there are as many octahedral gaps as there are packing particles in the closest packing of spheres, the empirical formula CaTiO 3 results again .
A number of other compounds crystallize in the cubic perovskite type , including industrially important ferroelectrics such as the above-mentioned barium titanate (BaTiO 3 ), but also other oxides such as CaZrO 3 or CaSnO 3 as well as fluorides and nitrides with compositions such as e.g. B. KNiF 3 , KMnF 3 and ThTaN 3 .
Other structures can also be derived from the perovskite structure. There is only one kind of A-atom in perovskite. If half of all A atoms are systematically exchanged (i.e. every second one) for an atom A ', one obtains the elpasolite structure (AA'B 2 O 6 ). Their unit cell corresponds to eight times that of perovskite.
If the central atoms surrounded by twelve oxygen atoms (i.e. the calcium atoms in the case of perovskite) are removed from the structure, another common type of structure arises, the rhenium (VI) oxide structure, which can be described as a defect structure variant of the cubic perovskite structure.
morphology
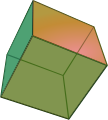
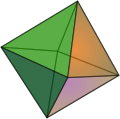
Perovskite most often crystallizes in cubic form , with the cubes being slightly distorted due to the orthorhombic symmetry. Octahedral or cuboctahedral single crystals are rarer . The octahedral surfaces , as well as some rhombic dodecahedron surfaces, are at least indicated in many cube-shaped crystals. The possible crystal shapes are shown below.
Modifications and varieties
In addition to calcium and titanium, perovskite can contain traces or even larger amounts of other metals . Instead of calcium, it can contain alkali metals , rare earth metals and, more rarely, iron ; niobium and subordinate tantalum and zirconium are often found on the titanium positions .
Varieties with a very high content of rare earth metals (especially cerium ) are called knopite , very niobium-rich perovskites are called dysanalytics , and a combination of both is also known as loparite . Taking into account the elements it frequently contains, the chemical composition of perovskite can also be given more generally as (Ca, Na, Fe 2+ , Ce, Sr) (Ti, Nb) O 3 .
Education and Locations
Perovskite is formed by crystallization from titanium-rich magmas and is a common component of low-silica ( mafic ) rocks such as syenite , kimberlite or carbonatite , but it also occurs in carbonate-rich metamorphic rocks such as skarn . Perovskite could also be detected in carbonate-containing chondrites ( stone meteorites ). The accompanying minerals ( paragenesis ) include nepheline (K, Na) AlSiO 4 , titanite CaTiSiO 5 , ilmenite FeTiO 3 , magnetite Fe 3 O 4 , and - particularly importantly - melilite : its occurrence indicates the occurrence of perovskite (and vice versa) .
There are numerous sites of perovskite worldwide, in addition to the type locality of Akhmatovsk and other places in the Urals , these include the Kola peninsula (Russia), the Eifel and the Kaiserstuhl (Germany), Zermatt (Switzerland) as well as the Val di Susa and Val Malenco (Italy).
Perovskite can be produced artificially at temperatures of more than 1300 ° C through the reaction of calcium oxide CaO and titanium dioxide TiO 2 with subsequent crystallization:
The melting point of the end product calcium titanate is 1975 ° C and thus slightly higher than that of titanium dioxide at 1843 ° C.
Occurrence in the earth's mantle
The dominant mineral phase of the lower mantle (between 660 and 2900 km depth) is also known as perovskite. It is an iron and magnesium-containing silicate with the composition (Mg, Fe) SiO 3 , which has the same crystal structure as CaTiO 3 perovskite. The magnesium-containing end member of the mixed crystal series of this silicate perovskite was only recently detected as a natural mineral in the Tenham meteorite and was officially named Bridgmanite in 2014 .
Use of materials with a perovskite structure
Perovskites such as barium titanate are used as ferroelectrics and, for example, as dielectrics in ceramic capacitors . With compounds derived from the perovskite structure, the later Nobel Prize winners Johannes Georg Bednorz and Karl Alexander Müller achieved the breakthrough in the novel ceramic high-temperature superconductors in 1986 . It was lanthanum-barium-copper oxides.
Suitable perovskites can be used to build light emitting diodes .
In addition, certain perovskites can be used for the production of solar cells . A working group led by Michael Graetzel achieved efficiencies of 15% on small prototypes under laboratory conditions. At the beginning of 2014, an efficiency of - officially unconfirmed - 19.3% was achieved under laboratory conditions. In 2017, Swiss researchers succeeded in increasing efficiency to over 22 percent. This efficiency is in the range of the best silicon-based solar cells (around 25 percent) in mass production. The problem with perovskites as a solar cell material is the amount of lead required for conventional perovskite cells , since if the use of lead in solar cells is prohibited in the EU under the RoHS directive, economic usability is in question. In addition, perovskite solar cells are sensitive to moisture and so far have a significantly shorter service life than solar cells made from other materials. With new research approaches these problems should be eliminated and efficiencies of 23% should be achieved. Although it is possible in principle to replace lead with tin , attempts of this kind have so far been largely unsuccessful, as tin gradually oxidizes and the crystal structure of the perovskite is lost. As of 2014, the efficiency of lead-free Perovskite cells based on a CH 3 NH 3 SnI 3 structure was a good 6%. A decisive step in the development of lead-free perovskite cells is the prevention of the oxidation of the tin content in the cell in order to ensure long-term stability. If this is successful, lead-free perovskite cells could be developed within a few years, which not only consist of non-toxic materials, but also have a higher degree of efficiency than lead-containing perovskite cells.
In implantology, perovskite enables the unusual combination of titanium or its oxide with the biological tissue of the bone: under biological conditions, perovskite (CaTiO 3 ) is formed on the surface of rough osseointegrated titanium implants . In this crystal, the bone's calcium is ionically bound rather than covalent, which was believed until recently. On the biological side, the positively charged calcium ion binds to the loci of the poly anions of the long-chain glycosamine glycans in the extracellular matrix, and on the crystal side of the implant surface to the covalently linked titanium-oxygen complex. The ionic binding type with its lower binding forces enables complete bone tissue remodeling also on the implant surface, while a covalent calcium bond to and in the TiO 2 crystal prevents the calcium ion from being freely available in the permanent remodeling process between bone tissue and the metal crystal.
The Eidgenössische Materialprüfungs- und Forschungsanstalt Empa is working in cooperation with the ETH Zurich on a perovskite-based color sensor that could enable higher resolutions and light sensitivity for digital cameras.
See also
literature
- WA Deer, RA Howie, J. Zussman: An Introduction to the Rock Forming Minerals . Prentice Hall, Harlow 1992, ISBN 0-582-30094-0 . (English)
- Will Kleber , Hans-Joachim Bautsch , Joachim Bohm : Introduction to crystallography . 18th edition, Verlag Technik, Berlin 1998, ISBN 3-341-01205-2 .
- U. Müller: Inorganic Structural Chemistry . 5th edition, Teubner, Stuttgart 2006, ISBN 3-8351-0107-2 .
- M. Okrusch, S. Matthes: Mineralogy . Springer, Berlin 2005, ISBN 3-540-23812-3 .
- Science magazine, special issue: Perovskites. November 17, 2017. Vol 358, Issue 6365. http://science.sciencemag.org/content/358/6364
Web links
- Mineral Atlas: Perovskite (Wiki)
- Perovskites. In: mindat.org. Hudson Institute of Mineralogy, accessed November 16, 2019 .
- David Barthelmy: Perovskite Mineral Data. In: webmineral.com. Retrieved November 16, 2019 .
- Perovskite structure (CaTiO 3 type, E2 1 type). In: ruby.chemie.uni-freiburg.de. University of Freiburg , February 13, 2017, accessed on November 16, 2019 .
Individual evidence
- ↑ a b c RH Buttner, EN Maslen: Electron difference density and structural parameters in CaTiO 3 . In: Acta Crystallographica . B48, no. 5 , 1992, pp. 644-649 , doi : 10.1107 / S0108768192004592 .
- ^ G. Rose: De novis quibusdam fossilibus, quae in montibus uraliis inveniuntur, scripsit. 1839, Berlin.
- ↑ G. Rose: About some new minerals of the Urals . In: Journal for practical chemistry. Vol. 19, 1840, pp. 459-460.
- ↑ YA Abramov, VG Tsirelson, VE Zavodnik, SA Ivanov, ID Brown: The chemical bond and atomic displacements in SrTiO 3 from X-ray diffraction analysis . In: Acta Crystallographica . B51, no. 6 , 1995, pp. 942-951 , doi : 10.1107 / S0108768195003752 .
- ↑ RH Buttner, EN Maslen: Structural parameters and electron density difference in BaTiO 3 . In: Acta Crystallographica . B48, no. 6 , 1992, pp. 764-769 , doi : 10.1107 / S010876819200510X .
- ^ VM Goldschmidt: The laws of crystal chemistry . In: The natural sciences . tape 14 , no. 21 , 1926, pp. 477-485 ( [1] ).
- ^ William Scott MacKenzie, C. Guilford: Atlas of rock-forming minerals in thin sections . Enke, Stuttgart 1981, ISBN 978-3-432-91911-9 , pp. 29 .
- ^ G. Pfaff: Synthesis of calcium titanate powders by the sol-gel process . In: Chemistry of Materials . tape 6 , 1994, pp. 58 , doi : 10.1021 / cm00037a013 (English).
- ↑ Bruce Dunn, Jeffrey I. Zink: Sol-Gel Chemistry and Materials . In: Accounts of Chemical Research . tape 40 , no. 9 , 2007, p. 729 , doi : 10.1021 / ar700178b , PMID 17874844 .
- ↑ Joanna Wendel. 2014. Mineral Named After Nobel Physicist. Eos, Transactions American Geophysical Union, Vol. 95, No. 23, p. 195; doi: 10.1002 / 2014EO230005 .
- ↑ Tan et al., Bright light-emitting diodes based on organometal halide perovskite. Nature Nanotechnology , August 3, 2014, doi: 10.1038 / nnano.2014.149 .
- ↑ Yuandi Li: Reducing the cost of perovskite solar cells. In: www.rsc.org. Chemistry World, April 18, 2013, archived from the original on May 2016 ; accessed on November 16, 2019 .
- ↑ J. Burschka, N. Pellet and a .: Sequential deposition as a route to high-performance perovskite-sensitized solar cells . In: Nature . tape 499 , no. 7458 , July 2013, ISSN 1476-4687 , p. 316-319 , doi : 10.1038 / nature12340 , PMID 23842493 (English).
- ^ A b Martin A. Green, Anita Ho-Baillie, Henry J. Snaith: The emergence of perovskite solar cells . In: Nature Photonics . tape 8 , no. 7 , 2014, ISSN 1749-4885 , p. 506-514 , doi : 10.1038 / nphoton.2014.134 (English).
- ↑ futurezone: Researchers achieve breakthrough in perovskite solar cells. In: futurezone.at. Retrieved November 16, 2019 .
- ↑ Boris Hänßler: Solar cells made from perovskite. Spektrum.de , December 13, 2013, accessed on November 16, 2019 .
- ↑ Perovskite Crystal: New Source of Solar Energy. August 14, 2014, accessed November 16, 2019 .
- ↑ Sascha Mattke: Photovoltaic researchers are working on efficient solar modules with silicon and perovskite. In: heise.de. Heise online , January 19, 2016, accessed November 16, 2019 .
- ^ Rui Wang et al .: Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells . At: Joule No. 3, 1-14 June 2019 doi: 10.1016 / j.joule.2019.04.005 .
- ^ Nicola Armaroli , Vincenzo Balzani : Solar Electricity and Solar Fuels: Status and Perspectives in the Context of the Energy Transition . In: Chemistry - A European Journal . tape 22 , no. 1 , 2016, p. 32-57 , doi : 10.1002 / chem . 201503580 .
- ↑ Nakita K. Noel et al .: Lead-free organic-inorganic tin halide perovskites for photovoltaic applications . In: Energy and Environmental Science . tape 7 , 2014, p. 3061-3068 , doi : 10.1039 / c4ee01076k .
- ↑ A. Wirthmann et al. Interaction between bones and titanium - new insights into the unusual “marriage” of bones and metal. Z Zahnaerztl Implantol 2014; 30: pp. 288-300.
- ↑ Karin Weinmann: The stacked color sensor. In: empa.ch. November 16, 2017. Retrieved August 20, 2019 .



![{\ mathrm {{} _ {\ infty} ^ {{3}} \ lbrace [TiO {} _ {{6/2}} ^ {{e}}] ^ {{2 -}} \ rbrace \! \ ,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b2e5fc1a02aa24cfb63d6f3719ef80e17d6c561f)