Niggli formula
The Niggli formula or Niggli notation was developed by the Swiss crystallographer Paul Niggli (1888–1953) and enables the representation of almost infinite connection patterns in the structure of crystals . The coordination numbers of the atoms are shown in the form of fractions and can thus be read directly from the formula. By adding up the individual fractions, the Niggli formulas give the sum formula of the compound , provided that all atoms are taken into account .
General explanations
Complete Niggli Formula
A complete Niggli formula with two different atoms A and B has the following form:
- The curly brackets mark the beginning and the end of the formula, the superscript number (n) indicates the dimensionality of the connection:
- n = 0 ( zero-infinity ) for insulated with each other unlinked units in the crystal structure (for example, single [SiO 4 ] - tetrahedra in island silicates )
- n = 1 ( one-infinite ): for one-dimensionally linked substructures (for example, [SiO 4 ] tetrahedra linked to chains in pyroxenes via two corners )
- n = 2 ( two-infinite ): for two-dimensional substructures (for example, [SiO 4 ] tetrahedra linked to surfaces via three corners in phyllosilicates )
- n = 3 ( three-infinite ): for three-dimensional partial structures or even complete structures (for example, [SiO 4 ] tetrahedra linked over all corners in structural silicates or quartz )
- The square brackets mark the (partial) structure described. Atom A is located in the center of the coordination polyhedron , which is formed from atoms B. The numbers x / y in brackets indicate the coordination numbers of the atoms to one another:
- Atom A has x contacts with atom B.
- Atom B has y contacts with atom A.
- The superscript letter after atom B (v) is optional. There you can specify what type of link it is:
- v = t ( terminal ): Atom B does not link to any other atom A or its coordination polyhedron
- v = e ( linked to corners): Atom B is connected to two atoms of type A, the coordination polyhedra of A have common corners
- v = k ( edge-sharing ): Two atoms B are connected to two atoms A, the coordination polyhedra of A have common edges
- v = f ( face- linked): At least three atoms B are connected to two atoms A, the coordination polyhedra of A have common faces
- The superscript number (m) at the end of the square brackets is also optional and, if available, indicates the charge of the formula part in the square brackets.
The Niggli formula, however, does not generally reveal the type of structure in which the compound is present or the shape of the coordination polyhedra of the particles. A 6-fold coordinated particle can be surrounded both in the form of an octahedron and as a trigonal prism .
Coordination number 6 as an octahedron
Coordination number 6 as a trigonal prism
Abbreviated Niggli formula
The formula from the example above has this abbreviated form:
In the case of abbreviated Niggli formulas, the curly brackets, information about the type of connection (v) and usually the charge (m) are usually left out. In the case that y = 1, y can be omitted:
Examples from crystal structures
Structures with isolated polyhedra (zero-infinity)
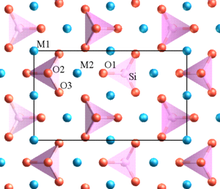
Zircon
The mineral zircon Zr [SiO 4 ] belongs to the mineral group of island silicates . The [SiO 4 ] tetrahedra in its crystal structure are not connected to one another and are isolated.
- Full Niggli Formula:
- Abbreviated Niggli formula:
- or
The isolated substructure has four negative charges, the zirconium - cations are therefore four times positively charged in zircon, the charge balance. In the empirical formula, there is a [SiO 4 ] 4− tetrahedron in Zr 4+ cation : Zr [SiO 4 ].
Structures with chain substructures (one-infinite)
Enstatite and wollastonite (chain silicates)
The chains of [SiO 4 ] tetrahedra in the chain silicates enstatite MgSiO 3 and wollastonite CaSiO 3 can be described with the same Niggli formula, although the chain motifs differ.
- Full Niggli Formula:
- Abbreviated Niggli formula:
- or
Adding the fractions results in “SiO 3 ”. While the motif in enstatite is repeated after two tetrahedra ( two-single chain ), this is only achieved in wollastonite after three tetrahedra ( three-single chain ). Such structural differences cannot be read from the Niggli formula, in both cases the chain is formed by two corner-linking oxygen atoms. In both cases, the chain carries a double negative charge, which is balanced in enstatite by the Mg 2+ and in wollastonite by the Ca 2+ cations.
Structures with surface (partial) structures (two-infinite)
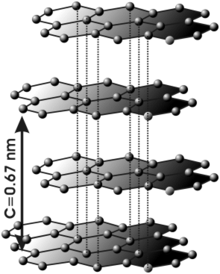
graphite
In the graphite modification of carbon , the carbon atoms form layers, with one carbon atom being covalently bonded to three others within the layer. The honeycomb structure of the layers consists of hexagons that share their edges with neighboring hexagons.
- Full Niggli Formula:
- Abbreviated Niggli formula:
Since it is elemental carbon, no charge appears in the full Niggli formula either. The sum formula here is C 2 , which can be reduced to C.
Cadmium iodide
The crystal structure of cadmium iodide CdI 2 contains [CdI 6 ] octahedra, each of which is linked to form surfaces via three common edges. Each cadmium atom is connected to six iodide ions, the iodide ions in turn have contact with three cadmium atoms.
- Full Niggli Formula:
- Abbreviated Niggli formula:
In this case, by reducing the fraction 6/3, the sum formula CdI 2 is obtained .
Structures with scaffolding structures (three-infinite)
Sodium chloride
The crystal structure of sodium chloride NaCl can be described as the closest cubic packing of sodium ions in which all octahedral gaps are occupied by chloride ions or vice versa. The structure thus contains [NaCl 6 ] octahedra, which are three-dimensionally linked to one another over all corners or [ClNa 6 ] octahedra linked to all corners . Since both types of ions form a cubic closest packing of spheres with the other type of ion in the octahedral gaps, the crystal structure of NaCl is also called a commutative partial lattice . The Niggli formula can also be reversed in this example and leads to the same result.
- Full Niggli Formula:
- or.
- Abbreviated Niggli formula:
- or.
The Niggli formula results in the sum formula NaCl (in sum formulas the cations are placed in front of the anions).
literature
- Ulrich Müller: Inorganic Structural Chemistry . 5th edition. BG Teubner Verlag / GWV Fachverlage GmbH, Wiesbaden 2006, ISBN 3-8351-0107-2
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {n} \ lbrace [AB {} _ {x / y} ^ {v}] ^ {m} \ rbrace \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d1ed6c02e9a4fdb66a57c50d32e44e107326834b)


![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {n} [AB_ {x / y}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a825e8e6bf603b54f698b8e88b6f30477350e217)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {n} [AB_ {x}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/852d6a489bbbf299ba93fae909aa6fd3af1b28df)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {0} \ lbrace [SiO {} _ {4/1} ^ {t}] ^ {4 -} \ rbrace \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/74a86d253c8d37ac499b442f185d470aff0cb9ef)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {0} [SiO_ {4/1}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7fc9890ef667f2f1d17014f47c6df9cdbf8441e2)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {0} [SiO_ {4}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b6904edd2aaa6c7fead50c0477fe1d6821b87efd)

![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {1} \ lbrace [SiO_ {2/2} ^ {e} O_ {2/1} ^ {t}] ^ {2-}} \ rbrace}](https://wikimedia.org/api/rest_v1/media/math/render/svg/78900e29f9050d5e917415996d619819ef7e255a)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {1} [SiO_ {2/2} O_ {2/1}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5ea8b0c0238275dee30a1c879598fcf8eabd960f)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {1} [SiO_ {2/2} O_ {2}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0da8f1783baf96538bdb11b583ed33f227a0f27c)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {2} \ lbrace [CC {} _ {3/3} ^ {k}] \ rbrace \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0aa15e4bad60227035a9f872433d8e24cfe0ee7f)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {2} [CC_ {3/3}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ed16c7c0744b9f52052343132068a8449be0eb76)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {2} \ lbrace [CdI {} _ {6/3} ^ {k}] \ rbrace \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/acd4204d2d4acc8f7030642bd3b92f38e995a343)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {2} [CdI_ {6/3}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/31a1851a03bc5a0ed0e4275e2b840fd522fa81eb)



![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {3} \ lbrace [NaCl {} _ {6/6} ^ {e}] \ rbrace \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/60ec0c299c57c66f60b96cd258e4cae0a46ab56a)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {3} \ lbrace [ClNa {} _ {6/6} ^ {e}] \ rbrace \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e02628da9359be8b61553707738916a59d9b55d8)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {3} [NaCl_ {6/6}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0397bc94e86abfa93f0373d11cd30e0b83315668)
![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {3} [ClNa_ {6/6}] \! \,}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/44e48446920cfd9e22bf0d532c08ebeccded05f9)