Zirconium
| properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Zirconium, Zr, 40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 4 , 5 , d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-67-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-176-9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.342 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.021% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 91.224 (2) u | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 155 (206) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 148 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Kr ] 4 d 2 5 s 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 6th.634 126 (5) eV ≈ 640.1 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 13.13 eV ≈ 1 267 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 23.170 (4) eV ≈ 2 236 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 34.41836 (6) eV ≈ 3 320.87 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 80.348 (7) eV ≈ 7 752 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modifications | two (α- / β-Zr) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal ; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 6.501 g / cm 3 (25 ° C ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 1.1 · 10 −4 ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2130 K (1857 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 4650 K (4377 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 14.02 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 591 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 16.9 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | 0.00168 Pa at 2125 K. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 4650 ( long. ), 2250 ( trans. ) Ms −1 at 293.15 K. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 270.0 J kg −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 2.36 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 22.7 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −1.553 V (ZrO 2 + 4 H + + 4 e - → Zr + 2 H 2 O) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.33 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zirconium , often also zirconium , is a chemical element with the element symbol Zr and the atomic number 40. Its name is derived from zircon , the most common zirconium mineral . In the periodic table it is in the 5th period ; it is the second element of the 4th group (obsolete 4th subgroup ) or titanium group . Zirconium is a very corrosion-resistant heavy metal . Biological functions are not known; it occurs in small amounts (4 mg / kg) in the human organism and is not toxic .
history
The important zirconium- containing mineral zircon (Zr [SiO 4 ]) has been known as a gemstone since ancient times . Zirconium as an element was discovered in 1789 by Martin Heinrich Klaproth in a sample of the mineral zircon from Ceylon and named after it. The metal was first presented in 1824 by Jöns Jakob Berzelius by reducing K 2 ZrF 6 with potassium . To do this, he heated “a mixture of hydrofluoric zirconium-potassium with potassium in an iron tube” . After treatment with water, drying and prolonged heating with dilute hydrochloric acid , Berzelius obtained a “lumpy powder that was black like coal” and only “obtained a dark gray color and shine when pressed together with the polishing steel” . The correct atomic mass , on the other hand, could not be determined until 1924 because - besides errors in the implementation of the experiments - it was not known that zirconium always contains small amounts of hafnium . Without this information, measurements always gave a slightly too high atomic mass. The first practical application of zirconium was as a smokeless flash light powder .
Occurrence
Zirconium occurs in the earth's crust with a content of approx. 0.016%. In the list of elements , sorted by frequency , zirconium ranks 18th and is more common than the better-known elements chlorine and copper . Although it is very widespread, it is usually only found in very small quantities and in very small crystals (typically around 0.1 mm). This is why zirconium was considered rare in the past. Zirconium is found primarily in silicate intrusive rocks such as granite . It does not occur naturally, but only in some minerals , especially as zircon (ZrSiO 4 ) and baddeleyite (ZrO 2 ) as well as the rarer red eudialyte (Na 4 (CaCeFeMn) 2 ZrSi 6 O 17 (OHCl) 2 ). It is almost always associated with hafnium . Due to its high melting point of 2550 ° C, its great hardness and low reactivity, zircon is the oldest mineral that can be found on earth and can be used for radiometric age determinations due to the embedded uranium and thorium isotopes .
Secondary deposits, so-called soap deposits, are mostly used as raw materials . These occur when the surrounding rock is weathered and only the particularly weather-resistant zircon remains. Other such deposits can arise from water currents that wash out the zirconium crystals and wash them up in other places. Primary deposits, on the other hand, usually have a zirconium content that is too low for profitable mining.
The main zirconium deposits are in Australia , the USA and Brazil . With minable reserves of 38 million tons, the world annual production of zirconium minerals in 2006 was 920,000 tons (calculated as zircon). Only about 5% of this is processed into metal and alloys. The most important producing countries in 2006 were by far Australia and South Africa . According to the USGS , the world annual production of zirconium minerals in 2013 was 1.5 million t, of which 850,000 t in Australia. Other important producing countries were South Africa (170,000 t) and China (150,000 t). The prices for zirconium were USD 2,650 per ton in 2012 and USD 1,050 per ton in 2013.
Extraction and presentation

Zircon, the most common zirconium raw material, has to be converted into zirconium dioxide before further processing . For this purpose, the zirconia is in a sodium hydroxide - melt cooked (alkaline digestion ). The zirconium dioxide is then converted into zirconium carbonitride (carbon and nitrogen-containing zirconium) in an electric arc with coke and then with chlorine to form zirconium tetrachloride .
A direct reduction of zirconium dioxide with carbon (as in the blast furnace process ) is not possible, since the carbides formed are very difficult to separate from the metal . Instead, zirconium tetrachloride is reduced to zirconium metal in the so-called Kroll process with magnesium in a helium atmosphere.
The Van Arkel de Boer process is used to obtain purer zirconium . During the heating under vacuum, the zirconium initially reacts with iodine to form zirconium (IV) iodide . This is broken down again to zirconium and iodine on a hot wire:
Zirconium tetraiodide is formed from zirconium and iodine at 200 ° C; it disintegrates again at 1300 ° C.
Zirconium and hafnium cannot be separated in a simple chemical way. This is why this high-purity zirconium still contains hafnium. Since it is important for many applications in reactor technology that the zirconium no longer contains hafnium, separation processes for these two metals play an important role. One possibility are extraction processes in which the different solubility of zirconium and hafnium compounds in special solvents is used. The thiocyanates and their different solubility in methyl isobutyl ketone are often used . Ion exchangers or the fractional distillation of suitable compounds offer further possibilities .
The USGS states the US import price for zirconium as 75 USD per kg in 2013.
properties
Physical Properties
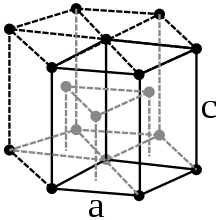
Zirconium is a silvery, shiny heavy metal ( density 6.501 g / cm 3 at 25 ° C), it looks similar to steel . The metal crystallizes in two different modifications , into which it can be converted by changing the temperature. Below 870 ° C, α-zirconium crystallizes in the hexagonal crystal system (hexagonal close packing of spheres, magnesium type) in the space group P 6 3 / mmc (space group no.194) with the lattice parameters a = 323 pm and c = 514 pm as well two formula units per unit cell . At 870 ° C the crystal structure changes to the body-centered cubic β-structure ( tungsten type) with the space group Im 3 m (No. 229) and the lattice parameter a = 361 pm.
Zirconium is relatively soft and pliable. It can be easily processed by rolling , forging and hammering . Small amounts of hydrogen , carbon or nitrogen impurities in the metal make it brittle and difficult to process. The electrical conductivity is not as high as that of other metals. It is only about 4% of that of copper . Due to its poor electrical conductivity, zirconium is a relatively good conductor of heat . Compared to the lighter homologue titanium , the melting and boiling points are slightly higher (melting point: titanium: 1667 ° C, zirconium: 1857 ° C). The electrical and thermal conductivity are also better. Below 0.55 K, zirconium becomes superconducting .
The properties of zirconium and the heavier homologue hafnium are very similar due to the lanthanide contraction . This requires similar atomic radii (Zr: 159 pm, Hf: 156 pm) and thus similar properties. The two metals, however, differ considerably in their density (Zr: 6.5 g / cm 3 , Hf: 13.3 g / cm 3 ).
An important property, because of which zirconium has gained great importance in reactor construction, is its small capture cross-section for neutrons . In this capacity, zirconium is also very different from hafnium. This makes the complex separation process necessary for these applications.
Chemical properties
Zirconium is a base metal that reacts with many non-metals , especially at high temperatures . Mainly as a powder, it burns with a white flame to form zirconium dioxide, and in the presence of nitrogen also to form zirconium nitride and zirconium oxynitride . Compact metal only reacts with oxygen and nitrogen when it is white heat . At increased pressure, zirconium reacts with oxygen even at room temperature, since the zirconium oxide formed is soluble in the molten metal. When zirconium is burned in oxygen, a temperature of approx. 4660 ° C is reached.
Zirconium is passivated in the air by a thin, very dense layer of zirconium oxide and is therefore inert. It is therefore insoluble in almost all acids , only aqua regia and hydrofluoric acid attack zirconium at room temperature. Aqueous bases do not react with zirconium.
Isotopes
Many isotopes between 78 Zr and 110 Zr are known of zirconium . Natural zirconium is a mixed element that consists of a total of five isotopes. These are 90 Zr, which occurs most frequently with a share of 51.45% of natural zirconium, as well as the heavier isotopes 91 Zr (11.32%), 92 Zr (17.19%), 94 Zr (17.28%) ) and 96 Zr with 2.76% share. 96 Zr is the only natural isotope that is weakly radioactive ; it decays with a half-life of 24 · 10 18 years with double beta decay to 96 Mo. The isotope 91 Zr can be detected with the aid of NMR spectroscopy .
88 Zr has a very large capture cross-section for thermal neutrons . Overall, after 135 Xe, it is the second largest so far determined effective cross-section for the capture of thermal neutrons. The value is about 80,000 times greater than the theoretical prediction suggests.
use
An important use for zirconium are made of Zircaloy made shells of uranium - fuel in nuclear power plants . This alloy consists of approx. 90% zirconium and a small amount of tin , iron , chromium or nickel , but must not contain any hafnium . The reason for the choice of this element is the small cross-section for thermal neutrons already described above and at the same time great corrosion resistance , which also makes it suitable as a building material for chemical plants, especially for special apparatus parts such as valves , pumps , pipes and heat exchangers . As an alloy additive to steel, it also increases corrosion resistance. Surgical instruments, among other things, are made from these alloys.
Since zirconium reacts with small amounts of oxygen and nitrogen, it can be used as a getter material in incandescent lamps and vacuum systems to maintain the vacuum. This property is also used in metallurgy to remove oxygen, nitrogen and sulfur from steel .
Because of its property of emitting a very bright light when burned, it was used as a flashlight powder in addition to magnesium . In contrast to magnesium, zirconium has the advantage of being smoke-free. This property is also used in fireworks and signal lights .
Zirconium emits a surge of sparks when it hits metal surfaces and is flammable. The military uses this in some types of ammunition, such as the specialty shotgun ammunition Dragon's Breath and the US-American all-purpose cluster ammunition BLU-97 . In film technology , this effect is used for non- pyrotechnic impact effects of, for example, bullets on metal surfaces.
Zirconium- niobium alloys are superconducting and remain so when strong magnetic fields are applied. They were therefore used earlier for superconducting magnets .
safety instructions
There are no known toxic effects of zirconium and its compounds. Because of the dense oxide layer , compact zirconium is not flammable. In powder form, however, it can start to burn when heated in the air. Zirconium fires are very dangerous because neither water (violent reaction with formation of hydrogen ), nor carbon dioxide or halon can be used to extinguish them. Zirconium fires must be extinguished with metal fire extinguishers (class D) or dry sand.
proof
With alizarin red -S, zirconium forms a characteristic red-violet compound (colored varnish) in acid, which disappears again when fluoride ions are added to form the zirconium fluorocomplex. This reaction can serve as qualitative evidence of both zirconium and fluorine. Since even small amounts of fluoride (and other anions) interfere, this detection is unsuitable for mineral analyzes. In addition, some other organic compounds, such as tannin , copperron , phenylarsonic acid , oxine or xylenol orange , are suitable as detection reagents. Another characteristic compound is zirconium oxychloride ZrOCl 2 · 8 H 2 O, which crystallizes in typical needles. In modern analytics , zirconium can be detected using atomic absorption spectrometry (AAS) or mass spectrometry (also using the isotope pattern ).
One possibility for quantitative analysis is the precipitation of sparingly soluble zirconium (IV) hydroxide with ammonia and subsequent incineration to zirconium dioxide.
-
- Precipitation of the hydroxide
-
- Convert to weighing form
links
→ Category: Zirconium compound
As a base metal, zirconium forms a multitude of compounds . Most zirconium compounds are salts . They are often very stable and have a high melting point. The + IV oxidation state is preferred and the most stable. But there are also compounds in the oxidation states + III to + I, and in complexes even in the states 0, −I and −II.
Zirconia
The most important zirconium compound is zirconium dioxide ZrO 2 , a very stable and high-melting oxide. Zirconium dioxide is used to make refractory linings in crucibles and furnaces . In order to use it for this, however, it must be stabilized with calcium , yttrium oxide or magnesium oxide to stabilize the cubic high-temperature phase . Zirconia -reinforced aluminum oxide ( ZTA , Zirconia Toughened Aluminum Oxide ) is used as technical ceramics for high temperatures.
Zirconia crystals are colorless and have a high refractive index . That is why they are used under the name zirconia as artificial gemstones and substitutes for diamonds . In addition, zirconium dioxide is used as an abrasive and, because of its white color, as a white pigment for porcelain .
If zirconium oxide is doped with yttrium oxide , further application possibilities arise. At three percent yttrium oxide content, the ZrO 2 is stabilized in a distorted fluorite structure. As a result, it acts as a conductor for oxygen ions at temperatures of over 300 ° C. An important application for this is the lambda probe in cars, which is used to measure the oxygen content in exhaust gases for the catalytic converter . With 15% yttrium oxide content, zirconium oxide emits a very bright, white light at 1000 ° C. This is used in the so-called Nernst lamp . Since yttrium-zirconium ceramics have an extremely high fracture toughness , they are used, for example, in dental technology as a highly stable crown and bridge framework, in artificial hip joints and dental implants or as a connecting element in telescopes . They are increasingly replacing gold and other metals in their function.
Zirconia is also often used for ball bearings . Especially for the bearing races, ZrO 2 has the great advantage that the coefficient of thermal expansion is close to that of steel. Other technical ceramics such as silicon nitride usually have a considerably lower coefficient of thermal expansion.
Halides
With the halogens fluorine , chlorine , bromine and iodine , zirconium forms several series of compounds. Compounds of the forms ZrX 4 , ZrX 3 and ZrX 2 are known from all halogens . In addition there are the chlorides , bromides and iodides of the form ZrX. The tetrahalides of the form ZrX 4 are the most stable . No important areas of application are known for any of the zirconium halides, with zirconium chlorides being formed as intermediate products in the production of pure zirconium.
Other zirconium compounds
Zirconium silicate , ZrSiO 4 , better known under the mineral name zircon , is the most common zirconium compound found in nature. It is the most important source of zirconium and its compounds. Zircon is also used as a gemstone .
Organic zirconium compounds are mostly unstable. Organic zirconium complexes , so-called zirconocenes , with radicals such as cyclopentadienyl are of particular importance . They are technically important as a catalyst in the polymerization of alkenes , especially for the production of polypropylene . Another application of an organic zirconium compound is in hydrozirconation . Alkenes are converted into alcohols or halogenated hydrocarbons with the aid of the Schwartz reagent Cp 2 ZrHCl (Cp = cyclopentadienyl) . In the reaction of terminal alkynes with the Schwartz reagent, trisubstituted double bonds are formed during hydrozirconation; further reaction with an electrophilic reagent leads to trans-functionalized alkenes in high stereochemical purity.
Aluminum- zirconium complexes can be used as an antiperspirant . Potassium hexafluoridozirconate (IV) K 2 ZrF 6 (CAS number: 16923-95-8) can be used to separate zirconium from hafnium.
Zirconium carbonate exists as a basic complex. It is used, among other things, in the paper industry .
In addition to aluminum-containing alums , zirconium salts are used in the "white tanning" of skins .
Lead zirconate titanate ceramics (PZT ceramics) are used for piezo elements .
Sodium zirconium cyclosilicate is a non-absorbable powder with a microporous structure that can absorb potassium in exchange for hydrogen and sodium cations. It is therefore used medicinally as an orally available potassium binder.
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- Hans Breuer: dtv-Atlas Chemie. Volume 1, 9th edition. dtv-Verlag, 2000, ISBN 3-423-03217-0 .
- Michael Binnewies: General and Inorganic Chemistry. 1st edition. Spektrum Verlag, 2004, ISBN 3-8274-0208-5 .
- NN Greenwood, A. Earnshaw: Chemistry of the Elements. 1st edition. VCH Verlagsgesellschaft, 1988, ISBN 3-527-26169-9 .
Web links
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values of the atomic and physical properties (info box) are taken from www.webelements.com (Zirconium) .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on zirconium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on zirconium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ A b c Gordon B. Skinner, Herrick L. Johnston: Thermal Expansion of Zirconium between 298 ° K and 1600 ° K. In: J. Chem. Phys. 21, 1953, pp. 1383-1284, doi: 10.1063 / 1.1699227 .
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure. The sign in this source is likely to be a printing error, since transition metals are fundamentally paramagnetic (see second source).
- ↑ H. Kojima, RS Tebble, DEG Williams: The variation with temperature of the magnetic susceptibility of some of the transition elements . In: Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences. 260 (1301), 1961, pp. 237-250. Values there are based on the mass in grams and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure. In contrast to the previous source, the sign is positive here.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ a b c Entry on zirconium, powder, not stabilized in the GESTIS substance database of the IFA , accessed on August 9, 2016(JavaScript required) .
- ↑ Entry on zirconium in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ^ Johann Joseph Prechtl: Yearbooks of the imperial royal polytechnic institute in Vienna. Volume 9, 1826, p. 265.
- ↑ O. Hönigschmied, E. Zintl, F. Gonzalez: About the atomic weight of zirconium. In: Journal of General and Inorganic Chemistry. 139, 1924, pp. 293-309.
- ^ Hans Breuer: dtv-Atlas Chemie. Volume 1, 9th edition. dtv-Verlag, 2000, ISBN 3-423-03217-0 .
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. 1988, ISBN 3-527-26169-9 , p. 1231.
- ↑ Data sheet zirconium from Acros, accessed on 19 February 2010. .
- ↑ Mineral Yearbook 2005 of the US Geological Society for zirconium (PDF; 158 kB).
- ↑ Zirconium at usgs Mineral Resources (PDF; 62 kB).
- ↑ a b MINERAL COMMODITY SUMMARIES 2015. (PDF 2.3 MB) USGS , pp. 191–192 (188–189) , accessed on September 7, 2015 (English).
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH Verlagsgesellschaft, 1988, ISBN 3-527-26169-9 .
- ↑ Entry on zirconium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2019.
- ↑ G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties. In: Nuclear Physics. Volume A 729, 2003, pp. 3-128. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ↑ Spectrum of Science: Nuclear Physics Record: Radioactive Exotic Turns Out to be Neutron Scare - Spectrum of Science , accessed on March 9, 2019
- ^ Helmut Hofmann, Gerhart Jander: Qualitative analysis. de Gruyter, Berlin 1972, p. 147.
- ^ H. Lohninger: Zirconium . In: Inorganic Chemistry. Retrieved April 23, 2014.
- ↑ Gerhard Jander, Ewald Blasius: Introduction to the inorganic chemical practical course (qualitative analysis). 13th edition. S. Hirzel Verlag, Stuttgart 1990, p. 130.
- ^ Ordinance on the analysis of cosmetics (PDF; 920 kB).
- ↑ Entry on oxide ceramics. In: Römpp Online . Georg Thieme Verlag, accessed on April 4, 2014.
- ↑ High-performance ceramics from CEROBEAR .
- ↑ Jörg Zimpel: Industrial and commercial wastewater discharges into public wastewater systems: Requirements and problem solutions. expert-Verlag, 1997, ISBN 3-8169-1421-7 , p. 195.
- ↑ Sheridan M. Hoy: Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia . In: Drugs . tape 78 , no. October 15 , 2018, p. 1605–1613 , doi : 10.1007 / s40265-018-0991-6 , PMID 30306338 , PMC 6433811 (free full text).