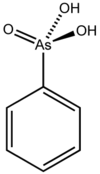
Phenylarsonic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phenylarsonic acid | ||||||||||||||||||
| other names |
Benzaric acid |
||||||||||||||||||
| Molecular formula | C 6 H 7 AsO 3 | ||||||||||||||||||
| Brief description |
white odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 202.04 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.760 g cm −3 |
||||||||||||||||||
| Melting point |
158 ° C (decomposition) |
||||||||||||||||||
| solubility |
slightly soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Phenylarsonic acid is a chemical compound from the group of arsenic acid derivatives in which a hydroxyl group has been replaced by a phenyl group .
Extraction and presentation
Phenylarsonic acid can be synthesized in several ways, but is mostly made by reacting phenyldiazonium salts with sodium arsenite in the presence of copper (II) catalysts.
It was first made by Michaelis and Loenser.
properties
Phenylarsonic acid is a flammable white odorless solid that is sparingly soluble in water. It decomposes above 158 ° C.
use
Phenylarsonic acid is used as a starting material for other organic arsenic compounds, some of which, e.g. B. Roxarson and sodium hydrogen arsenic , are used in animal nutrition.
It is also used as a precipitation reagent for tetravalent metal ions such as tin (IV), zirconium (IV) and thorium (IV).
Individual evidence
- ↑ a b c d e f g Entry on phenylarsonic acid in the GESTIS substance database of the IFA , accessed on January 15, 2020(JavaScript required) .
- ↑ Bullard, RH; Dickey, JB: Phenylarsonic Acid In: Organic Syntheses . 15, 1935, p. 59, doi : 10.15227 / orgsyn.015.0059 ; Coll. Vol. 2, 1943, p. 494 ( PDF ).
- ↑ A. Michaelis, H. Loesner: About nitrated phenyl arsenic compounds . In: Reports of the German Chemical Society . 27, 1877, pp. 263-272. doi : 10.1002 / cber.18940270151 .
- ↑ A. Michaelis: About aromatic arsenic compounds . In: Reports of the German Chemical Society . 8, 1875, pp. 1316-1317. doi : 10.1002 / cber.187500802125 .
- ↑ A. Michaelis, W. La Coste, A. Michaelis: About the compounds of the elements of the nitrogen group with the radicals of the aromatic series. Third treatise: On aromatic arsenic compounds . In: Annals of Chemistry . 201, No. 2-3, 1880, pp. 184-261. doi : 10.1002 / jlac.18802010204 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 810.


