Arsanilic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
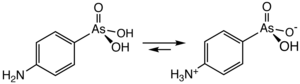
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Arsanilic acid | |||||||||||||||||||||
| other names |
p -arsanilic acid |
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
crystalline powder (sodium hydrogen arsenilate) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 217.05 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
> 300 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Arsanilic acid , more precisely p- arsanilic acid (formerly brand name Atoxyl ), is a chemical compound from the group of organic arsenic acid derivatives (more precisely the derivatives of phenylarsonic acid ). Their esters and salts are known as arsanilates.
properties
Arsanilic acid is a derivative of phenylarsonic acid with an amino group in the 4-position. It exists as a zwitterion , H 3 N + C 6 H 4 AsO 3 H - , although it is usually described with the non-zwitterionic formula.
history
Arsanilic acid was first prepared in 1863 by Antoine Béchamp by reacting aniline with arsenic acid .
Béchamp chose the name Atoxyl for his arsenic anilide to indicate its lower toxicity compared to arsenic . Initially, Atoxyl was only used externally against skin diseases.
In 1906, the German physician and Nobel Prize winner Robert Koch discovered on an expedition to Africa that Atoxyl can also have a beneficial effect on dangerous sleeping sickness . However, the effect was not very pronounced, or the required dosage was so high that the toxic side effects of the arsenic preparation, which led to blindness and even death, predominated.
Nevertheless, this discovery was later an important basis for the bacteriologist Paul Ehrlich in the development of arsphenamine , the first modern drug for the treatment of syphilis caused by spirochetes . Ehrlich had tested Atoxyl, influenced by the work of Paul Uhlenhuth and other scientists, as a drug against syphilis, but soon realized that he had misjudged the chemical structure of the preparation.
Sodium 4-aminophenyl hydrogen arsonate
The sodium salt of arsanilic acid, sodium 4-aminophenyl hydrogen arsonate , was tested as an arsenic-based active ingredient at the beginning of the 20th century .
Historical literature
- Atoxyl. In: Naturarzt. Volume 37, 1909, p. 268.
Individual evidence
- ↑ a b c Entry on Sodium 4-Aminophenylarsonate at TCI Europe, accessed on June 27, 2011.
- ↑ a b c d e data sheet p-Arsanilic acid from Sigma-Aldrich , accessed on November 7, 2016 ( PDF ).
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry arsenic compounds, with the exception of those named in this appendix in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A. Béchamp: De l'action de la chaleur sur l'arséniate d'aniline et la formation d'une anilide de l'acide arsénique . In: Comptes rendus des séances de l'Académie des sciences . Volume 56, 1863, pp. 1172-1175.
- ^ Wolfgang U Eckart: Illustrated history of medicine . Springer, 2010, ISBN 978-3-642-12609-3 ( page 308 in the Google book search).
- ↑ Steven Riethmiller: From Atoxyl to Salvarsan. Searching for the magic bullet. In: Chemotherapy. Volume 51, 2005, pp. 234-242, here: p. 239.
- ↑ Florian G. Mildenberger : No salvation through arsenic? The salvarsand debate and its consequences. In: Specialized prose research - Border Crossing 8/9, 2012/2013, pp. 327–390, here: pp. 332 f.
- ↑ Lutz Heuer: Milestone in the pharmaceutical industry - The Bayer plant in Elberfeld until 1923 Deutsche Apotheker Zeitung 2018, No. 11, p. 82, March 15, 2018.




