Titanium (IV) oxide
| Crystal structure | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Titanium (IV) oxide in the modification rutile __ Ti 4+ __ O 2− |
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Titanium (IV) oxide | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Ratio formula | TiO 2 | |||||||||||||||||||||
| Brief description |
white crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 79.866 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
|
|||||||||||||||||||||
| Melting point |
1855 ° C |
|||||||||||||||||||||
| boiling point |
2900 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
optically anisotropic , birefringent or biaxial |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 3 mg m −3 (measured as respirable dust ) |
|||||||||||||||||||||
| Toxicological data |
> 5.5 mg l −1 ( LC 50 , crustaceans , 48 h ) |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Titanium (IV) oxide ( titanium dioxide ) is the IV- valued oxide of titanium . In addition to this polymorphic oxide, there are a number of non-stoichiometric suboxides of titanium, so-called Magneli phases, as well as titanium (III) oxide and titanium (II) oxide .
Titanium dioxide has a wide range of uses as a white pigment , which is why four to five million tons are produced worldwide every year. The main areas of application are in the area of coatings such as lacquers and paints, followed by plastic coloring and laminate papers. Colored products usually also contain white pigments in order to achieve high hiding power.
The use of titanium dioxide as a food additive is controversial. In May 2021, the European Food Safety Authority (EFSA) designated the use of the substance listed as E 171 as “unsafe”. France had already banned its use in food in 2020; Switzerland also wants to follow suit by the end of 2021.
history
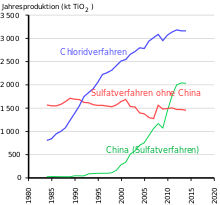
After William Gregor discovered titanium in the ilmenite in 1791 , Heinrich Klaproth recognized titanium dioxide in rutile . Industrial use began when its excellent suitability as a white pigment was recognized in Norway and the USA in 1908 . From 1916 the pigment was produced commercially under the name Kronos Titan White. Until 1938, titanium white was only produced in the anatase modification, but then increasingly in the rutile modification, since its photocatalytic activity is lower and the weathering stability of the products made from it is correspondingly higher. The white pigment based on the rutile modification is also known as rutile white.
More than half of the production volume is used in coatings, followed by polymers and paper. In 2014, 70% of world production was produced by five manufacturers in the western world. In addition to the market leader Chemours (USA, previously DuPont ), these are the companies Cristal Global (taken over by Tronox in 2019, Saudi Arabia), Tronox (USA), Venator Materials (United Kingdom, formerly part of Huntsman , USA) and Kronos (USA) . The largest manufacturer in Asia is LomonBillions (People's Republic of China). According to a presentation by Venator Materials from June 2018, the five largest western manufacturers had 54% of the global production capacity. The regions that consume the most titanium dioxide are Europe (1.72 million t), China (1.42 million t), the rest of Asia (1 million t) and North America (0.89 million t).
Occurrence
Titanium (IV) oxide occurs naturally in four modifications :
- Rutile is a tetragonal mineral with a mostly prismatic habit . The crystal structure is in the space group 136, which corresponds to the Hermann Mauguin symbol P4 2 / mnm. The rutile TiO 2 has a density of 4.26 g / cm 3 . The name rutile comes from the Latin rutilus 'reddish', alluding to the color produced by iron impurities.
- Anatase forms tetragonal holohedral crystals (holohedral means the most symmetrical group within a crystal system ) in the tetragonal thus 4 / m 2 / m 2 / m . It crystallizes in space group 141, that is I4 1 / amd. Anatase converts irreversibly into rutile at 700 ° C, depending on the atmosphere and foreign ions. The density of anatase is 3.88 g / cm 3 .
- Brookite forms orthorhombic minerals and crystallizes in space group 61, Pbca. Brookite also changes to rutile below the melting point and has a density of 4.12 g / cm 3 . Technically, the brookite has no meaning.
- Riesite ( IMA 2015-110a ) is a high pressure modification that crystallizes in the monoclinic crystal system in the space group P 2 / c (space group no.13 ) and was discovered in 2015 by Oliver Tschauner and Chi Ma in Nördlinger Ries (Bavaria, Baden-Württemberg) .
Further modifications
In addition to the natural modifications, eight synthetically produced modifications are known, three of which are metastable (monoclinic, tetragonal and orthorhombic) and five high-pressure modifications (α-PbO 2 , baddeleyite, cotunnite and orthorhombic and cubic structures). The modification with a cotunnite structure was described by L. Dubrovinsky et al. as the hardest known oxide with a Vickers hardness of 38 G Pa and a compression modulus of 431 GPa (for comparison: diamond has 442 GPa to 446 GPa) under normal pressure. Later studies came to different results with lower values for the hardness (7–20 GPa, thus softer than oxides such as corundum Al 2 O 3 and rutile) and the compression modulus (≈ 300 GPa).
| modification | Crystal system | Manufacturing |
|---|---|---|
| TiO 2 (B) | monoclinic | Hydrolysis of K 2 Ti 4 O 9 with subsequent tempering |
| TiO 2 (H), hollandite -like structure form | tetragonal | Oxidation of the potassium titanate bronze, K 0.25 TiO 2 |
| TiO 2 (R), ramsdellite- like structure | orthorhombic | Oxidation of the lithium titanate bronze Li 0.5 TiO 2 |
| TiO 2 (II) - ( α-PbO 2 -like structure) | orthorhombic | |
| Baddeleyite -like structure, (7-fold coordinated Ti) | monoclinic | |
| TiO 2 -OI | orthorhombic | |
| cubic structure | cubic | P > 40 GPa, T > 1600 ° C |
| TiO 2 -OII, cotunnite ( PbCl 2 ) -like structure | orthorhombic | P > 40 GPa, T > 700 ° C |
Extraction and presentation
Titanium dioxide may be in the laboratory by hydrolysis of Ti (IV) compounds such as titanyl sulfate , titanium tetrachloride as metal alcoholates or titanium tetraisopropylate are prepared:
- Reaction of titanium oxide sulfate with water to form titanium oxohydrate and sulfuric acid
- Reaction of titanium tetrachloride with water in the first step to titanium oxychloride and hydrochloric acid and then to titanium oxohydrate and hydrochloric acid
- Titanium tetraisopropoxide and water react to form titanium dioxide and isopropanol
Since the titanic acid esters of the lower n -alkanols react too violently, the use of isopropanol or tert- butanol esters is recommended. The titanium oxohydrate obtained in this way, formally TiO (OH) 2 or TiO 2 ×H 2 O, is converted into anatase or rutile by calcination , with pure, highly annealed titanium dioxide always producing the rutile lattice. The combustion of titanium (IV) chloride with oxygen is rarely used on a laboratory scale. Very pure titanium dioxide can be produced by hydrolyzing purified TiCl 4 .
Since most of the industrially produced TiO 2 is used as a pigment, coloring ions such as iron interfere . Ilmenite (FeTiO 3 ) or titanium-containing slags from the electroreduction of ilmenite are generally used as ores for the sulphate process . This slag, just like rutile from alluvial deposits , can also be used in the technically more demanding chloride process. Both processes significantly increase the purity of the titanium oxide. The sum of the coloring ions is usually less than 200 ppm in the sulphate process, mainly niobium, subordinate to iron, and less than 50 ppm in the chloride process, niobium and iron.
In the industrial production of titanium oxide from ilmenite using the sulphate process, dilute acid (dilute sulfuric acid) is formed, which is mostly reused after concentration for the ilmenite digestion . In some countries this dilute acid is still partly discharged into rivers and seas or dumped . The extraction by the chloride process , mainly from rutile ore or TiO 2 slag, on the other hand, does not allow the formation of thin acid. The used chlorine remains largely in the process cycle. The iron salts produced in both processes are used, among other things, for chromate reduction in cements, wastewater treatment and in biogas plants.
Single crystals
Single crystals of rutile are usually made using the Verneuil process . The zone melting process is also occasionally used, while the Czochralski process is described as unsuitable.
Anatase single crystals cannot be produced from the melt. Here are CTR used method.
properties
Physical Properties
The melting point of titanium dioxide is 1855 ° C, the compound is thermally stable. Titanium dioxide is also chemically inert . It is lightfast, inexpensive and therefore the most important white pigment. It is approved as an additive E171 for food.
Optical properties
The refractive index of titanium oxide is high, and it shows a large dispersion . The refractive index also depends significantly on the crystal modification. Titanium dioxide is birefringent . The difference in the refractive index between an ordinary ray and an extraordinary ray can reach a value of up to .
From a coloristic point of view, due to its high refractive index, titanium dioxide has the highest hiding power of all white pigments and, at the same time, excellent lightening power . The maximum coverage of titanium dioxide is at a grain size of about 200 nm to 300 nm depending on the application and reference, number-based or mass-based size distribution.
Titanium dioxide is a semiconductor , so the valence band is fully filled and the conduction band unoccupied at zero temperature . The band gap depends on the modification. Light quanta with an energy greater than the band gap are absorbed. UV light can also be absorbed from the appropriate wavelength, thus creating UV protection. Short-wave light irradiation lifts electrons from the valence band into the conduction band and leaves a hole . The size of the band gap depends on the crystal direction and, in the area of nanoparticulate material, also on the particle size.
| modification | Band gap (eV) | Wavelength (nm) | interpolated index of refraction at 589 nm |
|---|---|---|---|
| Anatase | 3.23 | 385 | n e = 2.489 n o = 2.561 |
| Brookite | 3.14 | 395 | n α = 2.585 n β = 2.583 n γ = 2.702 |
| Rutile | 3.02 | 410 | n e = 2.900 n o = 2.613 |
Dielectric properties
Titanium dioxide has a comparatively high dielectric constant . For rutile it is ε = 111 in the crystallographic a-direction and ε = 257 along the c-axis. Other sources give smaller values, whereby the values depend on measurement parameters such as frequency and temperature. Applications are, for example, high-k dielectrics .
Chemical properties
Of the titanium oxides, titanium (IV) dioxide is the most common compound. It is chemically inert and can only be dissolved in hot sulfuric acid, hydrofluoric acid and hot alkalis. It is partly the starting material for the production of titanates . When illuminated with UV light, photocatalytic radical reactions can take place.
use
Titanium dioxide is mainly used as a white pigment and is listed in the Color Index under CI Pigment White 6 or CI 77891. It is chemically stable and is used under the E 171 label as a food additive, for example as a brightener for toothpaste, chewing gum, confectionery, cheese or sauces and as a separating agent (see section Risks ). Titanium dioxide pigments are used as CI 77891 in cosmetics. It is also partly used in oil painting . The technical fields of application of titanium dioxide, which account for around 80 percent of total consumption, include paints and varnishes , plastics and textiles; It is also used in paper production to achieve a high degree of whiteness and as a UV blocker in sun creams and brighteners in pharmaceuticals (tablets).
pigment
Titanium dioxide has a refractive index that is significantly higher than that of most organic substances that are used to bind colors. This means that pigments made from titanium oxide scatter the light effectively, resulting in a well-covering white color. The optimum size of the pigments is in the range from 200 nm to 300 nm. The size range results from the Mie theory . The particle size influences the opacity on the one hand and the color tone on the other; finely divided pigments appear more bluish. The most important applications are coating materials with a market share of around 60% and polymer applications.
Except as E171, pure titanium dioxide is rarely used, since in addition to the UV protective effect of the TiO 2, light-induced chemical radical reactions take place. Functionalization of the pigment grains reduces this effect and at the same time improves the color properties, usually through easier dispersion. Some applications, e.g. B. for fibers or cement applications, use anatase pigments despite the higher photochemical activity, while the majority of applications fall back on rutile pigments.
Photocatalyst
Many manufacturers offer photocatalysts based on TiO 2 . These are usually anatase, anatase-rutile mixtures or doped titanium dioxides with a wide range of possible applications. Photocatalysis is a heterogeneous catalysis in which gaseous or dissolved substances react under UV lighting by radical reaction or charge carrier transfer to titanium dioxide or other substances. By illuminating with UV light, the energy of which is greater than the band gap, or by the less efficient excitation from the impurities of a doping, free charge carriers, electrons in the conduction band and holes in the valence band are generated. As a rule, these charge carrier pairs recombine very quickly, but the bending of the band in the area of the surface can result in charge carrier separation. These usually react with adsorbed oxygen and water to form hydroxyl and peroxy radicals. As a rule, except in the case of direct charge transfers to adsorbates, the radicals react with adsorbed organic substances. The reaction pathways up to complete mineralization can be very complex and require many photon excitations.
In the case of outdoor use, photocatalytic self-cleaning as an example, the UV component of sunlight ASTM 1.5 of around 3% is generally used , a maximum of around 35 W / m 2 . Indoor applications are usually less favorable, on the one hand the UV component is very low or the reaction rate is low in the case of doped catalysts. The parameters in photocatalysis are differently defined quantum yields . Typical values can hardly be given, since a large number of parameters go into the catalysis. Usually orders of magnitude of 1 reaction per 1000 photons are mentioned. Another problem is that the photocatalytic reactions do not differentiate between the organic binder matrix and the pollutants. Unsuitable binder systems therefore tend to chalk early .
Other uses
In the production of special optical glasses, TiO 2 is used to influence the optical dispersion, the Abbe number . Titanium dioxide in the anatase modification is the main component of the catalysts used for the industrial denitrification of flue gases using the SCR process . The dye solar cell ( Grätzel cell ) is based on the semiconductor properties of titanium dioxide . With the help of titanium dioxide, memristors were made . Titanium dioxide is also used as the main component of the ceramic dielectric in class 1 ceramic capacitors . Synthetic rutile single crystals are used for optical prisms or as diamond imitations due to their optical properties . The imitations are easy to recognize due to the birefringence. In addition, titanium dioxide is used to produce test aerosols .
proof
Titanium dioxide freshly precipitated in the cold is amphoteric and soluble in dilute mineral acids. A digestion takes place with potassium hydrogen sulfate in a porcelain crucible. Then it is dissolved in cold water with a little sulfuric acid. With a few drops of hydrogen peroxide, the yellow (basic) to yellow-orange (acidic, photo) [Ti (O 2 ) · aq] 2+ cation is formed.
Dating
In stratigraphic investigations on 115-year-old Viennese light rail railings painted around 15 times by Otto Wagner , the first appearance of rutile white was used to date paint layers.
Risks
Titanium dioxide is not classified as hazardous to water .
In June 2017, ECHA's Risk Assessment Committee (RAC) assessed the classification proposal of the French authority, which had proposed classification and labeling as “probably carcinogenic in humans” (Carc 1B), and came to the conclusion that titanium dioxide was suspected of being carcinogenic if inhaled is to be classified (Carc 2). This classification proposal had to be checked by the European Commission and implemented in applicable law.
Very high concentrations of nanoparticles , i.e. particles with less than 100 nm, lead to immune reactions in the lungs . The immune reaction is discussed with the possibility of an inflammation-based cancer risk, with nanoparticulate TiO 2 smaller than 100 nm being tested and pigmented TiO 2 larger than 200 nm being used as an example application and for the production quantity.
In a group of 56 people who were selectively chosen because of problems with titanium implants, 21 people showed a positive reaction in the MELISA test (lymphocyte transformation test ) with TiO 2 , while all 54 people in the group tested using the patch test , tested negative. A study by the University of North Carolina found that titanium dioxide nanoparticles were toxic to microglial brain cells in mice.
In tests by biologists at the University of Koblenz-Landau with daphnia (water fleas), some significant effects were found despite low titanium dioxide concentrations in the water: The concentrations used in the test, up to 2 mg / l, were up to a factor of over 1000 above that Suspected concentration in the environment of ng / l to a few µg / l. The primary effect was through the accumulation of particles on the chitin shell of the water fleas with fatal consequences in the test group. The next generation of daphnia also showed damage in the studies via a mechanism of action that was not interpreted and analyzed. Some of these studies are in direct contradiction to an older study with significantly higher concentrations of up to 50 mg / l.
In rats, after 100 days of oral titanium dioxide administration , the INRA gave i.a. Intestinal inflammation noted. According to the authors, the dose of 10 mg / kg corresponds to the amount that people can be exposed to through use as a food coloring E171. Another study also shows that bowel inflammation can be aggravated by E171.
Investigations into the cytotoxicity of photocatalytically active titanium dioxide nanoparticles showed that nanoparticles made of titanium dioxide can form reactive radicals by absorbing UV radiation, which are able to break down many organic substances. This property has numerous industrial applications, but it also carries the risk of harmful effects on living organisms.
Classification as "probably carcinogenic if inhaled"
| safety instructions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Surname |
Mixtures in the form of powder with a content of at least 1% titanium dioxide in particle form or incorporated in particles with an aerodynamic diameter of ≤ 10 μm |
|||||||
| CAS number |
13463-67-7 |
|||||||
| EC number |
236-675-5 |
|||||||
|
||||||||
| Toxicological data |
> 5.5 mg l −1 ( LC 50 , crustaceans , 48 h ) |
|||||||
In 2012, titanium (IV) oxide was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health or the environment are re-evaluated and, if necessary, follow-up measures are initiated. The causes of the uptake of titanium (IV) oxide were concerns about other hazard-related concerns as well as the suspected dangers of carcinogenic properties and the possible danger of mutagenic properties. The reassessment started in 2018 and was carried out by France . After titanium dioxide caused inflammation in animal experiments and researchers suspected carcinogenic properties, France decided in 2019 to ban its use in food from 2020.
A potential health hazard is seen primarily in the inhalation of dusts; this has been the subject of numerous discussions. In October 2019, the EU Commission decided to classify and label titanium dioxide in powder form with at least 1% particles with an aerodynamic diameter ≤ 10 μm as presumably carcinogenic for humans (Category 2) through inhalation (H350 i). On February 18, 2020, the proposed classification of titanium dioxide was adopted as part of the 14th ATP (Adaptation to technical progress) in Regulation (EU) No. 2020/217 and is therefore to be implemented by October 1, 2021.
Use as a food additive
In May 2021, the European Food Safety Authority (EFSA) classified the dye as not safe for human consumption, as it could not rule out a negative effect of titanium dioxide on the human genome . In Switzerland, where foods containing titanium dioxide have had to bear the note nano in the list of ingredients since May 1, 2021 , E 171 will be banned as a food additive until the end of 2021, according to the Federal Food Safety and Veterinary Office ; some manufacturers already voluntarily dispense with the controversial dye in certain products.
Web links
- Zusatzstoffe-online.de: Titanium dioxide
- Photocatalytic self-cleaning using the example of wastewater treatment . Dissertation: Physico-chemical investigations on the mineralization of active pharmaceutical ingredients on titanium dioxide (PDF; 11.46 MB)
Individual evidence
- ↑ Entry on E 171: Titanium dioxide in the European database on food additives, accessed on June 16, 2020.
- ↑ Entry on TITANIUM DIOXIDE in the CosIng database of the EU Commission, accessed on August 5, 2020.
- ↑ a b c d e f g h i Entry on titanium (IV) oxide in the GESTIS substance database of the IFA , accessed on December 19, 2019(JavaScript required) .
- ↑ a b Entry on titanium dioxide. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b T. Radhakrishnan: The optical properties of titanium dioxide . In: Proceedings of the Indian Academy of Sciences - Section A . tape 35 , no. 3 , 1952, pp. 117-125 , doi : 10.1007 / BF03172227 .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 13463-67-7 or titanium dioxide ), accessed on November 2, 2015.
- ↑ Univ. Freiburg lecture script chemistry, oxides Part 4: Non-stoichiometric binary oxides.
- ↑ United States Geological Survey USGS TITANIUM MINERAL CONCENTRATES (English PDF; 27 kB).
- ↑ a b EFSA: Titanium dioxide: E171 is no longer considered safe when used as a food additive. May 6, 2021, accessed May 11, 2021 .
- ↑ a b Peter Fritsche: Controversial whitener - Switzerland also bans titanium dioxide in food. Swiss Radio and Television (SRF), May 11, 2021, accessed on May 11, 2021 .
- ↑ T. Brock, M. Groteklaes, P. Mischke; Paint technology textbook; 2nd Edition; Vincentz Network; Hanover; 2000; ISBN 3-87870-569-7 ; P. 123.
- ↑ https://www.icis.com/explore/resources/news/2018/07/19/10243097/tio2-players-in-major-asset-shuffle/
- ↑ Chemours: TiO2 Market Consumption (p. 9), Key Competitors and Technology (p. 13) , September 2015 (data status 2014)
- ↑ a b c Crystal structure database of the Center for Computational Materials Science ( Memento of the original from April 11, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. of the US Naval Research Laboratory .
- ↑ U. Hålenius, F. Hatert, M. Pasero, SJ Mills: IMA Commission on New Minerals, Nomenclature and Classification (CNMNC). Newsletter 35 . In: Mineralogical Magazine . tape 81 , no. 1 , February 2017, p. 209–213 ( main.jp [PDF; 79 kB ; accessed on August 19, 2017]).
- ↑ a b LS Dubrovinsky, NA Dubrovinskaia, V Swamy, J Muscat, NM Harrison, R Ahuja, B Holm, B Johansson: Materials science: The hardest known oxide . In: Nature . 410, No. 6829, 2001, pp. 653-654. doi : 10.1038 / 35070650 . PMID 11287944 .
- ↑ Oganov AR, Lyakhov AO: Towards the theory of hardness of materials . In: J. of Superhard Materials . 32, No. 3, 2010, pp. 143-147. doi : 10.3103 / S1063457610030019 .
- ↑ Y. Al-Khatatbeh, KKM Lee and B. Kiefer: High-pressure behavior of TiO 2 as determined by experiment and theory . In: Phys. Rev. B . 79, No. 13, 2009, p. 134114. doi : 10.1103 / PhysRevB.79.134114 .
- ↑ Nishio-Hamane D., Shimizu A., Nakahira R., Niwa K., Sano-Furukawa A., Okada T., Yagi T., Kikegawa T .: The stability and equation of state for the cotunnite phase of TiO 2 up to 70 GPa . In: Phys. Chem. Minerals . 37, No. 3, 2010, pp. 129-136. doi : 10.1007 / s00269-009-0316-0 .
- ↑ Marchand R., L. Brohan, Tournoux M .: A new form of titanium dioxide and the potassium octatitanate K 2 Ti 8 O 17 . In: Materials Research Bulletin . 15, No. 8, 1980, pp. 1129-1133. doi : 10.1016 / 0025-5408 (80) 90076-8 .
- ↑ M. Latroche, L. Brohan, R. Marchand, M. Tournoux: New hollandite oxides: TiO 2 (H) and K 0.06 TiO 2 . In: Journal of Solid State Chemistry . 81, No. 1, 1989, pp. 78-82. doi : 10.1016 / 0022-4596 (89) 90204-1 .
- ↑ J. Akimoto, Y. Gotoh, Y. Oosawa, N. Nonose, T. Kumagai, K. Aoki, H. Takei: Topotactic Oxidation of Ramsdellite-Type Li 0.5 TiO 2 , a New Polymorph of Titanium Dioxide: TiO 2 ( R) . In: Journal of Solid State Chemistry . 113, No. 1, 1994, pp. 27-36. doi : 10.1006 / jssc.1994.1337 .
- ↑ PY Simons, F. Dachille: The structure of TiO 2 II, a high-pressure phase of TiO 2 . In: Acta Crystallographica . 23, No. 2, 1967, pp. 334-336. doi : 10.1107 / S0365110X67002713 .
- ↑ Sato H., Endo S, Sugiyama M, Kikegawa T, Shimomura O, Kusaba K: Baddeleyite-Type High-Pressure Phase of TiO 2 . In: Science . 251, No. 4995, 1991, pp. 786-788. doi : 10.1126 / science.251.4995.786 . PMID 17775458 .
- ↑ Dubrovinskaia NA, Dubrovinsky L S., Ahuja R, Prokopenko V B., Dmitriev V., Weber H.-P., Osorio-Guillen JM, Johansson B: Experimental and Theoretical Identification of a New High-Pressure TiO 2 Polymorph . In: Phys. Rev. Lett. . 87, No. 27 Pt 1, 2001, p. 275501. doi : 10.1103 / PhysRevLett.87.275501 . PMID 11800890 .
- ↑ Mattesini M, de Almeida JS, Dubrovinsky L., Dubrovinskaia L, Johansson B., Ahuja R .: High-pressure and high-temperature synthesis of the cubic TiO 2 polymorph . In: Phys. Rev. B . 70, No. 21, 2004, p. 212101. doi : 10.1103 / PhysRevB.70.212101 .
- ↑ a b c Georg Brauer (Ed.): Handbook of Preparative Inorganic Chemistry . 3., reworked. Edition. tape II . Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1366 .
- ↑ C. Murty, R. Upadhyay, S. Asokan: Eletro Smelting of Ilmenite for Production of TiO 2 Slag. (PDF; 715 kB).
- ↑ Djeva company brochure on breeding using the Verneuil method (German, 4.2 MB PDF).
- ↑ Kazuhito Hatta, Mikio Higuchi, Junichi Takahashi, Kohei Kodaira, "Floating zone growth and characterization of aluminum-doped rutile single crystals", Journal of Crystal Growth, 163, 1996, pp. 279-284; doi: 10.1016 / 0022-0248 (95) 00972-8 .
- ↑ H. Machida and T. Fukuda: "Difficulties encountered during the Czochralski growth of TiO 2 single crystals", Journal of Crystal Growth, 112, 1991, pp. 835-837; doi: 10.1016 / 0022-0248 (91) 90142-R .
- ↑ T. Sekiya and S. Kurita, "Defects in Anatase Titanium Dioxide", Nano- and Micromaterials-Advances in Materials Research, 2008, Volume 9, pp. 121–141, doi : 10.1007 / 978-3-540-74557- 0_4 .
- ^ Polymers, Light, and the Science of TiO 2 DuPont . (1.42 MB PDF).
- ↑ Erik Shepard Thiele: Scattering of electromagnetic radiation by complex microstructures in the resonant regime . 1998 ( PDF, 3.2 MB - Ph.D. Thesis, University of Pennsylvania).
- ↑ J. Winkler: Titanium dioxide. Vincentz Network, Hannover 2003, ISBN 3-87870-738-X , p. 55.
- ↑ Michael Graetzel, Francois P. Rotzinger: The Influence of the Crystal Lattice Structure on the Conduction Band Energy of Oxides of Titanium (IV) . In: Chemical Physics Letters . Vol. 118, No. 5, 1985, pp. 474-477.
- ↑ Bora Lee, Choong-ki Lee, Cheol Seong Hwang and Seungwu Han: Influence of exchange-correlation functionals on dielectric properties of rutile TiO 2 , in: Current Applied Physics , Volume 11 (2011), S293-S296. doi : 10.1016 / j.cap.2010.11.104 .
- ↑ Rebecca A. Parker "Static Dielectric Constant of Rutile (TiO 2 ), 1.6-1060 ° K" , Phys. Rev. 124, 1961, pp. 1719-1722.
- ↑ Titanium dioxide in foods . Titanium Dioxide Manufacturers Association (TDMA), accessed May 11, 2021.
- ↑ Ceresana: Titanium Dioxide Market Study , accessed on May 21, 2013.
- ↑ KRONOS Titan: Areas of application for TiO 2 (PDF; 374 kB).
- ↑ Sachtleben: Hombikat UV 100 .
- ↑ Evonik: Aerosil, Aeroxid P25 ( Memento of the original from July 1, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ KRONOS Titan: Kronoclean data sheet (PDF; 488 kB).
- ↑ Neue Zürcher Zeitung: “Cobblestones against Smog” , overview article, November 16, 2011.
- ↑ Fraunhofer Photocatalysis Alliance: “Biological efficiency measurements for photocatalyst” (PDF; 79 kB).
- ↑ Tusnelda E. Doll: "Photochemical and photocatalytic degradation of carbamazepine , clofibric , iomeprol and iopromid ", Dissertation, 2004, DNB 1002433525/34 .
- ↑ Martin Lindner: "Optimization of the photocatalytic water purification with titanium dioxide: Solid and surface structure of the photocatalyst", dissertation, DNB 954460030/34 .
- ↑ Renewable Resource Data Center: Sunlight Spectrum ASTM 1.5 .
- ↑ Thorsten Ebbinghaus: "Combined biological-photocatalytic degradation of environmentally relevant nitrogen compounds for cleaning agricultural wastewater with overgrown plant filters and TiO 2 / UV" , TU Dortmund, dissertation, 2003 (PDF; 1.5 MB).
- ↑ Martin Klare: "Possibilities of the photocatalytic degradation of environmentally relevant nitrogen compounds using TiO 2 " , TU Dortmund, dissertation, 2003 (PDF; 3.3 MB).
- ↑ Kevin Bullis: Rewiring the Electronics . Technology Review. May 8, 2008, accessed March 25, 2010.
- ↑ VDI 3491 sheet 3: 2018-03 measurement of particles; Manufacturing process for test aerosols; Dispersion of piles and solids (Measurement of particles; Methods for generating test aerosols; Dispersing solid materials). Beuth Verlag, Berlin. P. 7.
- ↑ VDI 3491 sheet 9: 1989-09 measurement of particles; Production of test aerosols by means of a brush dispenser (Particulate matter measurement; generation of test aerosols with a rotating brush generator). Beuth Verlag, Berlin. P. 5.
- ↑ Otto Wagner and the Green of the 50s: Sehgewohnheiten und Reality orf.at, December 31, 2017, accessed December 31, 2017.
- ↑ ECHA press release of June 9, 2017
- ↑ nanopartikel.info: Nanocare , brochure, page 11 ff. (German, PDF; 2.7 MB).
- ↑ Yazdi, AS; Guarda, G .; Riteau, N .; Drexler, SK; Tardivel, A .; Couillin, I .; Tschopp, J. (2010). Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1 and IL-1 . Proceedings of the National Academy of Sciences 107 (45): pp. 19449-19454 doi: 10.1073 / pnas.1008155107 , PMC 2984140 (free full text).
- ↑ Biodenth.be (PDF; 245 kB) K. Müller, E. Valentine-Thon: "Hypersensitivity to titanium: Clinical and laboratory evidence" ( Memento of November 9, 2013 in the Internet Archive ) Neuroendocrinology Letters, Vol. 27, Suppl. 1, 2006, pp. 31-35.
- ↑ TC Long, N. Saleh, RD Tilton, GV Lowry, B. Veronesi: Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. In: Environmental Science & Technology . Volume 40, Number 14, July 2006, pp. 4346-4352. PMID 16903269 .
- ↑ Mirco Bundschuh, Frank Seitz, Ricki R. Rosenfeldt, Ralf Schulz, Elena A. Rozhkova: Titanium Dioxide Nanoparticles Increase Sensitivity in the Next Generation of the Water Flea Daphnia magna. In: PLoS ONE. 7, 2012, p. E48956, doi: 10.1371 / journal.pone.0048956 .
- ↑ Volker Mrasek : deutschlandfunk.de: Influence even across generations (March 22, 2014).
- ↑ Nadja Podbregar: Cross-generational consequences: nano-pollution of small crustaceans makes their offspring over-sensitive. In: scinexx.de. March 8, 2013, accessed August 20, 2019 .
- ↑ Kerstin Hund-Rinke, Markus Simon: Ecotoxic Effect of Photocatalytic Active Nanoparticles (TiO2) on Algae and Daphnids Environmental science and pollution research international: ESPR 13 (2006), No. 4, pp. 225-232, doi: 10.1065 / espr2006.06.311 .
- ↑ Bettini, S. et al .: Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Scientific Reports 7, Article number: 40373 (2017), doi: 10.1038 / srep40373 .
- ↑ Titanium dioxide nanoparticles can increase intestinal inflammation
- ↑ doi : 10.1136 / gutjnl-2015-310297
- ↑ C. Kasper, J. Z. Bloh, S. Wagner, D. W. Bahnemann, T. Scheper: Investigations on the cytotoxicity of photocatalytically active titanium dioxide nanoparticles. In: Chemical Engineer Technology. 82, 2010, p. 335, doi : 10.1002 / cite.200900057 .
- ↑ a b Entry on titanium (IV) oxide in powder form with at least 1% particles with an aerodynamic diameter ≤ 10 μm in the GESTIS substance database of the IFA , accessed on December 19, 2019(JavaScript required) .
- ↑ Entry on Titanium dioxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 7, 2021. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Titanium dioxide , accessed on March 26, 2019.
- ↑ France bans the controversial whitening agent titanium dioxide . Spiegel Online, April 17, 2019.
- ↑ Regulation (EU) 2020/217
- ↑ Titanium dioxide: There is still a need for research . Federal Ministry for Risk Assessment, July 29, 2020.
- ↑ Food safety: EU Commission proposes a ban on the approval of titanium dioxide. In: time online . May 6, 2021, accessed May 11, 2021 .
- ↑ Prohibit health-endangering nanoparticles as food additives. In: parlament.ch . 2019, accessed January 28, 2021 .
- ↑ Peter Fritsche: Controversial dye - titanium dioxide disappears from more and more products. Swiss Radio and Television (SRF), April 26, 2021, accessed on April 26, 2021 .









