Chalking
|
|
DIN EN ISO 4628-6 |
|---|---|
| Area | Coating materials |
| title | Assessment of coating damage - Assessment of the amount and size of damage and the intensity of uniform changes in appearance - Part 6: Assessment of the degree of chalking by the adhesive tape method |
| Latest edition | 2011-12 |
| ISO | 4628-6 |
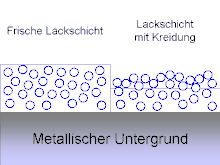
Chalking , chalks or chalking is a form of damage to with paint -coated surfaces. It shows the exposure of pigment and filler particles due to the degradation of the organic binder in the areas of the paint layer close to the surface. Chalking should not be confused with fading, i.e. the breakdown of colorants in the paint layer itself.
description
The damage caused by chalking occurs after the paint layer has been weathered. The binders contained in the paint are broken down more quickly near the surface than in deeper paint layers. Pigments and light stabilizers are evenly distributed within a paint layer, but develop a stronger effect the thicker the layer is. Layers lying deeper are therefore better protected than layers close to the surface.
As the binder breaks down near the surface, the other paint components such as pigments and fillers are exposed. This gives pigments and fillers an interface with air at the points previously wetted with binder . The colorless fillers in the paint appear white. Since the paint surface is no longer smooth, it appears matt. The higher scatter also looks brighter. Together, the typical whitish, matt appearance of chalked paint layers results.
If you wipe this surface, pigments and fillers are rubbed off. A whitish residue remains on the finger, similar to a layer of chalk, while the original color is visible again under the rubbed off surface.
Causes and countermeasures
In addition to the obvious cause of chalking, insufficient resistance of the binder used to UV light , the type of white pigment or fillers used in the formulation can also contribute to the chalking.
It is known that titanium dioxide pigments of the rutile modification are far more stable than titanium dioxide pigments of the anatase modification. In order to further minimize the influence, industrially used titanium dioxide types are often coated with zirconium dioxide , aluminum oxide , silicon dioxide , organic layers or combinations thereof. The exact process is described in the section Chalking cycle of titanium dioxide.
It is also known that the type of fillers used also plays a role in chalking. It was found that fillers with a high content of silicon dioxide such as mica , talc , kaolin or quartz are more critical than silicon dioxide-free types such as calcium carbonate , dolomite or barium sulfate .
Chalk cycle of titanium dioxide
In addition to the properties of the binder used, the effect of the chalking is also clearly influenced by the titanium dioxide usually contained in the formulation . The underlying processes are called the chalking cycle. They are based on the fact that titanium dioxide is a semiconductor .
- Electrons are raised to a higher energy level by absorption of UV light (charge separation), a positively charged gap remains in the valence band .
- The positively charged gap oxidizes a hydroxide ion . A hydroxyl radical is created . These radicals attack the binder matrix with the formation of further radicals. If the valence gap is occupied by an electron from the hydroxide ion, an excess of electrons remains in the conduction band of the titanium dioxide.
- The excess of electrons reduces Ti 4+ to Ti 3+ .
- The newly formed trivalent cation can be oxidized by oxygen . An oxygen radical anion is created, which is adsorbed on the surface of the titanium dioxide.
- If water is present, a hydroperoxide radical forms, which can interact with the polymer again , and a hydroxide ion, which reacts to form a hydroxyl group on the surface of the pigment.
From the chalking cycle it can be easily deduced why the weather fastness and light fastness of coatings do not correlate with one another. If no water is present, the reaction ends after the fourth sub-step.
exam
The degree of chalking of a coated surface can be determined using the adhesive tape method in accordance with the EN ISO 4628-6 standard . Here the chalking is assessed with characteristic values based on the intensity of the change in the surface, for example "Chalk 3 = very clear change". The assessment can be carried out both visually with comparison images and with digital image evaluation.
| Characteristic value | description |
|---|---|
| 0 | not changed, ie no noticeable change |
| 1 | very slight, ie just noticeable change |
| 2 | slight, ie clearly noticeable change |
| 3 | medium, ie very clearly perceptible change |
| 4th | strong, ie pronounced change |
| 5 | very strong change |
Deliberately chalking systems
Chalking is intentionally caused in self-cleaning emulsion paints . Strictly speaking, it takes advantage of the fact that the top layer of the coating is no longer bound to the rest and can therefore be easily removed. The individual recipe components are therefore coordinated in such a way that the chalking only takes place to a defined extent that is sufficient to remove the dirt lying on it. Since emulsion paints have a relatively thick layer and the surface is usually matt, the appearance and function of the coating are not affected by the chalking.
Web links
Individual evidence
- ↑ a b c d B. Müller; Additives compact ; Vincentz Network; 2008; ISBN 3866309155 .
- ↑ a b c d J. Winkler; Titanium dioxide ; Vincentz Network; Hanover; 2003; ISBN 387870738X .
- ↑ D. Gysau; Fillers ; Page 188ff; Vincentz Network; Hanover; 2005; ISBN 3878707932 .
- ↑ H. Römpp; Römpp Lexicon Lacquers and Printing Inks ; Page 336f; Thieme; Stuttgart; 1998; ISBN 9783137760016 .