Clavins
Clavins (also clavin alkaloids ) are tri- and tetracyclic chemical compounds belonging to the alkaloids . Clavins are produced by the ergot fungus Claviceps purpurea , which gives it its name , and other sac fungi of the genera Aspergillus , Claviceps , Balansia , Epichloë , Neotyphodium , Penicillium and Periglandula . Due to their occurrence in ergot and their chemical relationship to the other alkaloids derived from lysergic acid , they are assigned to the ergot alkaloids .
Occurrence
Clavines be especially sac fungi from the family of Clavicipitaceae formed. These include the representatives of the genera Claviceps , Balansia and Epichloë / Neotyphodium . The occurrence in ergot, the sclerotium of the ergot fungus Claviceps purpurea on rye and other sweet grasses, is of particular importance . In addition to the simple amides of lysergic acid and the ergot alkaloids of the peptide type, the clavins are also responsible for the toxicity of ergot.
Has long been the isolated occurrence of Clavinen and other ergot alkaloids in a surprisingly wind plants ( Convolvulaceae ), a phylogenetically sac fungus distant plant family considered. A possible infestation of the plants with ergot fungi was therefore discussed. Genetic studies have shown that a previously unknown genus of ergot fungus called Periglandula is responsible for the production of these alkaloids.
Without the involvement of ergot fungi, clavins can also be produced by some representatives of the Trichocomaceae family, which is not directly related to the Clavicipitaceae . Important producers of clavine alkaloids within this family are the representatives of the genera Aspergillus and Penicillium . There have also been isolated reports of the occurrence of clavins in other departments of the mushrooms, including the yoke and stand mushrooms .
structure
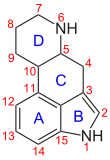
Clavins are indole alkaloids derived from the amino acid tryptophan . They have a tetracyclic basic structure, the ergoline basic structure, or a D-ring opened tricyclic basic structure (secoergoline) or a tri- or tetracyclic ring system created by alternative ring linking. Occasionally, alkaloids with an extended pentacyclic ring system and some bicyclic precursors of the biosynthesis of lysergic acid in connection with clavins are also mentioned. By definition, clavins differ from the other ergot alkaloids, which are derived from lysergic acid, by a lower oxidation level of the carbon atom , which corresponds to the substituent in position 8 of lysergic acid.
Secoergoline
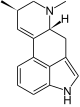
Tricyclic clavins, also known chemically as secoergolines, are intermediate products (and substances derived from them) in the biosynthesis of tetracyclic ergot alkaloids. The ring closure in ring D between N-6 and C-7 has not yet taken place in tricyclic clavins. The tricyclic clavins include, for example, the chanoclavins I and II, paliclavin , secolysergine and secoagroclavin .
Ergoline

|
|
| Chanoclavine I. | |
 |

|
| Festuclavin | Costaclavin |
 |

|
| Agroclavin | Elymoclavine |
 |

|
| Lysergine | Lysergol |
 |

|
| Setoclavine | Penniclavine |
 |

|
| Fumigaclavin A | Fumigaclavin C |
The tetracyclic clavins with an ergoline backbone represent the numerical majority within the clavine alkaloids family. They differ from one another in particular in the absence or presence of a double bond in ring D and the substituents in position 8 of the ergoline system.
Dihydroclavins
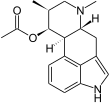
Dihydroclavins differ from the other ergot alkaloids in the lack of a double bond in ring D. Accordingly, dihydroclavins have three instead of two stereocenters . Festuclavin , its C-8 epimer pyroclavin and its C-10 epimer costaclavin occur in particular within the Clavicipitaceae . Further dihydroclavins, such as, for example, dihydroelymoclavine, could be isolated from cultures of Claviceps africana . The fumigaclavins from Aspergillus and Penicillium species are also among the dihydroclavins.
Δ 8.9 -ergolene
Δ 8,9 -ergolene are characterized by a double bond in the D-ring of the ergoline backbone between C-8 and C-9. The clavins with a Δ 8,9 -ergolen structure include, for example, agroclavin , elymoclavin and molliclavin .
Δ 9,10 -ergolene
Δ 9,10 -ergolenes have a double bond in the D ring of the ergoline backbone between C-9 and C-10. Among the Clavinen with a Δ 9,10 -Ergolenstruktur include Lysergin , Lysergen , lysergol , setoclavine and Penniclavin .
Substituents on C-8
In the case of dihydroclavins and clavins with a Δ 9,10 -ergolen skeleton (with the exception of lysergen), due to their center of asymmetry in position 8, a distinction can be made between substituents in 8α and 8β position. The methyl or hydroxymethyl group is in position 8β in the majority of the clavins of the Clavicipitaceae. Some clavins, such as setoclavin and penniclavin, have an additional hydroxyl group in position 8α . Clavins with an α-methyl or hydroxymethyl group are among the isoergolines. Examples are isolysergol, isopenniclavine and the isofumigaclavine.
Additional substituents
The tetracyclic clavins of the Clavipitaceae have, apart from substituents at C-8, a largely unmodified N -6-methylergoline or N -6-methylergole system. Only Molliclavin has a hydroxy group at C-9. The fumigaclavins, which are formed in particular by Aspergillus and Penicillium species, differ from most clavins of the Clavicipitaceae by additional substituents. Structurally, the fumigaclavins are derived from festuclavin. They have an additional hydroxy (fumigaclavin B) or acetoxy group (fumigaclavine A and C) on C-9 of the ergoline system. Fumigaclavin C also has an additional dimethylallylyl substituent at C-2 .
Another example of clavins with additional substituents is cividiclavin , a naturally occurring C-13-N'-1-pyroclavine dimer with a hydroxyl group at C-14 from Penicillium citreo-viride .
Alternative ring systems
Some alkaloids, which are biosynthetically related to classical clavins, have a tri-, tetra- or pentacyclic ring system that deviates from the secolergoline or ergoline skeleton and is formed by alternative ring linkages. The assignment of these alkaloids to the clavines is seen differently in the scientific literature.
In contrast to other tricyclic clavins, clavicipitic acid from Claviceps fusiformis and aurantioclavin from Penicillium aurantiovirens have a tricyclic azepinoindole backbone, which was created by alternative ring linking.
Also by alternative ring junction arise the tetracyclic 8-Oxergolin Paspaclavin from Claviceps paspali and in particular of various Penicillium produced TYPES Rugulavasine with their spiro Benzoindolfuran ring system.
In contrast, the relationship to classical clavins is clearly evident in the pentacyclic alkaloids epoxyagroclavin from Penicillium kapuscinski and cycloclavin from Aspergillus japonicus . Formally, they represent oxidation products of Agroclavins. In contrast, the structure of the pentacyclic cyclopiazonic acid from Penicillium cyclopium differs significantly from the ergoline structure.
biosynthesis
The biosynthesis of Clavine is by a series of enzymes , the so-called by a cluster EAS - Gene ( ergot alkaloid synthesis genes are encoded = Mutterkornalkaloidsynthesegene), catalyzed. Such a gene cluster could be detected in all the producers of clavins examined so far. Due to the commonality of many enzymes involved, the biosynthesis essentially proceeds across species according to a common scheme.
In a first reaction step, the amino acid tryptophan is prenylated to dimethylallyltryptophan with dimethylallylpyrophosphate with the participation of the gene product of dmaW , dimethylallyltryptophan synthase . A methyl transferase catalyzes the N -methylation to N -methyldimethylallyltryptophan. The C-ring closes to form chanoclavin-I with catalysis by chanoclavin synthase. A reduction in the hydroxyl group leads to secolysergin. An oxidation of chanoclavin-I, on the other hand, leads to chanoclavin-I-aldehyde, which is cyclized to agroclavin with the participation of the chanoclavin cyclase encoded by the easA gene and with ring closure of the D ring. A ring closure of the D-ring with the participation of a chanoclavin cyclase with reductase function leads to festuclavin and the fumigaclavins derived from it from Aspergillus fumigatus and dihydroclavins from Claviceps africana . Paspaclavin, on the other hand, is the product of an alternative D-ring ending.
An oxidation of the methyl group at C-8 of the Agroclavin to a hydroxymethyl group with the participation of a monooxygenase leads to the elymoclavin. A further oxidation of the substituent at C-8, which requires an intact cloA gene, would lead to the lysergic acids and the simple amides and peptides derived from them. Because of a defective cloA gene, the biosynthesis of claviceps fusiformis ends at the clavine stage. A direct oxidation at C-8 of Agroclavin and Elymoclavin with the participation of various peroxidases to setoclavin and penniclavin increases the variety of clavins.
properties
Clavins are part of the toxic principle of ergot and the psychotropic effect of the ritually used bindweed herbs ololiuqui (especially from Turbina corymbosa ) and tlitliltzin ( Ipomoea violacea and other Ipomoea species). Clavins convey their effects in particular via an interaction with dopamine and serotonin receptors as well as α 1 -adrenoceptors . They can act as moderately potent to potent antagonists or partial agonists at these receptors .
use
Clavine alkaloids themselves have no practical use. Some partially synthetic derivatives of ergot alkaloids, which can be formally regarded as clavins, are used in medicine. The dihydrolysergole derivative nicergoline is used to treat senile dementia . Pergolide , a thioether derived from dihydrolysergol, has been used in the therapy of Parkinson's disease . Metergoline , a carbamate of dihydrolysis ergamine , is used in veterinary medicine .
Individual evidence
- ↑ Schardl CL, Young CA, Hesse U et al .: Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci . In: PLoS Genet . 9, No. 2, 2013, p. E1003323. doi : 10.1371 / journal.pgen.1003323 . PMID 23468653 .
- ↑ Eckart Eich: Tryptophan-derived alkaloid . In: Solanaceae and convolvulaceae - secondary metabolites: biosynthesis, chemotaxonomy, biological and economic significance . Springer, 2008, ISBN 3540745408 , pp. 213-260.
- ↑ Steiner U, Leibner S, Schardl CL, Leuchtmann A, Leistner E: Periglandula, a new fungal genus within the Clavicipitaceae and its association with Convolvulaceae . In: Mycologia . 103, No. 5, 2011, pp. 1133-1145. doi : 10.3852 / 11-031 . PMID 21558502 .
- ↑ Anatoly G. Kozlovsky: Producers of ergot alkaloids out of the Claviceps genus . In: Vladimir Kren, Ladislav Cvak (eds.): Ergot: The Genus Claviceps. Medicinal and Aromatic Plants - Industrial Profiles . CRC Press, 2004, ISBN 0203304195 , pp. 479-499.
- ↑ Abe M, Yamatodan S: On a New Water-Soluble Ergot Alkaloid, Molliclavine . In: Bull. Agr. Chem. Soc . 192, 1955, pp. 161-162.
- ↑ Vining LC, McInnes AG, DG Smith, Wright JL Taber WA: Dimeric clavines alkaloid produced by Penicillium citreo viride . In: FEMS Symp . 13, 1982, pp. 243-251.
- ↑ Martin Buchta, Ladislav Cvak: Ergot alkaloids and other metabolites of the genus Claviceps . In: Vladimir Kren, Ladislav Cvak (eds.): Ergot: The Genus Claviceps. Medicinal and Aromatic Plants - Industrial Profiles . CRC Press, 2004, ISBN 0203304195 , pp. 173-201.
- ^ A b Wallwey C, Li SM: Ergot alkaloids: structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes . In: Nat Prod Rep . 28, No. 3, 2011, pp. 496-510. doi : 10.1039 / c0np00060d . PMID 21186384 .
- ↑ King GS, Waight ES, Mantle PG, Szczyrbak CA: The structure of clavicipitic acid, an azepinoindole derivative from Claviceps fusiformis . In: J. Chem. Soc., Perkin Trans . 1, 1977, pp. 2099-2103. doi : 10.1039 / P19770002099 .
- ^ Kozlovskiĭ AG, Solov'eva TF, Sakharovskiĭ VG, Adanin VM: [Biosynthesis of "unusual" ergot alkaloids by the fungus Penicillium aurantio-virens] . In: Dokl Akad Nauk SSSR . No. 1, pp. 230-233. PMID 7307906 .
- ↑ Tscherter H, Hauth H: Three new ergot alkaloids from saprophytic cultures of Claviceps paspali Stevens et Hall. 77. Communication on ergot alkaloids . In: Helvetica Chimica Acta . 57, No. 1, 1974, pp. 113-121. doi : 10.1002 / hlca.19740570111 .
- ↑ Holzapfel CW: The Isolation and structure of cyclopiazonic acid, a toxic metabolite of Penicillium cyclopium Westling . In: Tetrahedron . 24 pages = 2101-2119, 1968.
- ↑ Tudzynski P Hölter K, Correia T, Arntz C, N Grammel, cellar U: Evidence for ergot alkaloid of gene cluster in Claviceps purpurea . In: Mol Gen Genet . 261, No. 1, 1999, pp. 133-141. PMID 10071219 .
- ↑ Panaccione DG: Origins and significance of ergot alkaloid diversity in fungi . In: FEMS Microbiol Lett . 251, No. 1, 2005, pp. 9-17. PMID 16112823 .
- ↑ Cheng JZ, Coyle CM, Panaccione DG, O'Connor SE: Controlling a structural branch point in ergot alkaloid biosynthesis . In: J Am Chem Soc . 132, No. 37, 2010, pp. 12835-12837. doi : 10.1021 / ja105785p . PMID 20735127 .
- ↑ Lorenz N, Wilson EV, Machado C, Schardl CL, Tudzynski P: Comparison of ergot alkaloid biosynthesis gene clusters in Claviceps species indicates loss of late pathway steps in evolution of C. fusiformis . In: Appl Environ Microbiol . 73, No. 22, 2007, pp. 7185-2191. PMID 17720822 .
- ↑ Heinz Pertz, Eckart Eich: Ergot alkaloids and their derivatives as ligands for serotoninergic, dopaminergic, and adrenergic receptors . In: Vladimir Kren, Ladislav Cvak (eds.): Ergot: The Genus Claviceps. Medicinal and Aromatic Plants - Industrial Profiles . CRC Press, 2004, ISBN 0203304195 , pp. 411-440.