Indole alkaloids
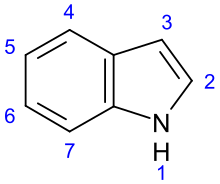
Indole alkaloids form the largest alkaloid group . It has well over 1000 representatives. They are characterized by the indole or indoline (2,3-dihydroindole) basic body. According to their biogenetic origin from tryptophan , almost all of them have a 2-aminoethyl residue in position 3 of the indole ring, which is mostly integrated into one or more rings fused to the indole .
Examples: Ajmaline , Ergotamine , Yohimbine , Reserpine , Strychnine
Some representatives are created by linking two substituted indole bodies. They are known as bis-indole alkaloids.
Occurrence in nature
Indole alkaloids come e.g. B. in the steppe rue ( Peganum harmala L.). This contains harmane alkaloids such as harmine , harmaline and tetrahydroharmine . The calabar bean ( Physostigma venenosum ) contains physostigmine . Some members of the Convolvulaceae plant family , such as B. Ipomoea violacea and Turbina corymbosa contain ergolines and lysergic acid amides . Many monoterpenoid indole alkaloids are found in three families of dicotyledonous plants: Apocynaceae ( Rauvolfia and Catharanthus ), Rubiaceae ( Corynanthe ), and Loganiaceae ( Strychnos ).
Indole alkaloids are also found in mushrooms. Mushrooms containing psilocybin contain derivatives of tryptamine and claviceps contains derivatives of lysergic acid . The skin of several real toads ( bufo ) also contains tryptamine derivatives ( bufotenin ). The skin of Bufo alvarius (a species of toad) contains 5-methoxy- N , N -dimethyltryptamine . Serotonin , an important neurotransmitter in many animal species, can be thought of as a simple indole alkaloid.
Rue contains β-carboline alkaloids
Ipomoea violacea contains ergoline
Rauwolfia serpentina contains Corynanthe alkaloids
Catharanthus roseus contains monoterpenoid indole alkaloids
Psilocybe cubensis contains psilocybin and psilocin
The toad Bufo alvarius contains bufotenin and 5-methoxy- N , N -dimethyltryptamine
Web links
Individual evidence
- ^ Hesse, Alkaloidchemie, Georg Thieme Verlag 1978 ISBN 3-13-381801-5
- ^ Albert Gossauer: Structure and Reactivity of Biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, pp. 480–482, ISBN 978-3-906390-29-1 .
- ↑ a b Waksmundzka-Hajnos, Monika; Sherma, Joseph; Kowalska, Teresa: Thin layer chromatography in phytochemistry . CRC Press, 2008, ISBN 978-1-4200-4677-9 , pp. 625-626.
- ↑ Tadeusz Aniszewski: Alkaloids - secrets of life . Elsevier, Amsterdam 2007, ISBN 978-0-444-52736-3 , p. 39.
- ↑ Waksmundzka-Hajnos, Monika; Sherma, Joseph; Kowalska, Teresa : Thin layer chromatography in phytochemistry . CRC Press, 2008, ISBN 978-1-4200-4677-9 , p. 626.
- ↑ Tadeusz Aniszewski: Alkaloids - secrets of life . Elsevier, Amsterdam 2007, ISBN 978-0-444-52736-3 , pp. 37-39.
- ↑ Michael E. Peterson, Patricia A. Talcott: Small Animal Toxicology . Saunders, 2005, ISBN 0-7216-0639-3 , p. 1086.
- ↑ Waksmundzka-Hajnos, Monika; Sherma, Joseph; Kowalska, Teresa : Thin layer chromatography in phytochemistry . CRC Press, 2008, ISBN 978-1-4200-4677-9 , p. 625.