Ergotamine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
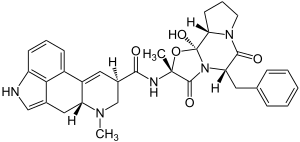
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Ergotamine | ||||||||||||
| other names |
(6a R , 9 R ) -7-methyl-4,6,6a, 7,8,9-hexahydroindolo [4,3- fg ] quinoline-9-carboxylic acid - [(2 R , 5 S , 10a S , 10b S ) -5-benzyl-10b-hydroxy-2-methyl-3,6-dioxooctahydrooxazolo [3,2- a ] pyrrolo [2,1- c ] pyrazin-2-yl] amide |
||||||||||||
| Molecular formula |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Melting point | |||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Ergotamine is a principal alkaloid of ergot and as drug used. It is a highly effective substance ( see LD 50 ), which is partly responsible for ergot poisoning ( Antonius fire ), among other things . Ergotamine can be used therapeutically for the acute therapy of migraines and for the short-term prophylaxis of cluster headaches .
Occurrence

The substance was isolated and described in 1918 by Arthur Stoll as the first pure ergot alkaloid . Ergotamine is obtained from the ergot fungus, which for this purpose z. B. is cultivated on rye .
chemistry
Chemically, ergotamine is a condensation product of lysergic acid and a tricyclic tripeptide made from alanine , phenylalanine and proline .
pharmacology
Ergotamine shows high (nanomolar and subnanomolar) affinities for α 1 - and α 2 - adrenoceptors , dopamine receptors and serotonin (5-HT) receptors . It acts as an agonist , partial agonist or antagonist at these receptors . The LD 50 value is 3.0 mg / kg ( rabbit , iv). The plasma half-life is 20–34 hours.
Its migraine effectiveness is based on the partial agonistic effect of ergotamine on 5-HT 1B / 1D on blood vessels in the central nervous system . Since ergotamine is problematic not only from a pharmacodynamic point of view (lack of selectivity), but also from a pharmacokinetic point of view (low, strongly fluctuating absorption , poor tissue penetration, relatively long half-life), it is regarded as the 3rd choice agent for the acute therapy of migraines.
The classic, old form of administration of ergotamine as a tablet by the mouth is actually very variable in effectiveness because of the very different absorption into the bloodstream and used to lead to abuse more often. However, if the route of administration is chosen via a rectiole , the effectiveness at a lower dose than normal - due to the rapid and almost complete absorption in the rectum - is overall safe and good; but there is also the risk of abuse and prolonged daily use of a perianal ulcer .
biosynthesis
Ergotamine is one of the secondary metabolites and is the essential alkaloid of the ergot fungus ( Claviceps purpurea ). The ergotamine biosynthesis in this and closely related fungi begins with the condensation of the amino acid , L - tryptophan by prenylation with dimethylallyl diphosphate, which by the enzyme dimethylallyl-tryptophan (DMAT) synthase catalyzes is. This first biosynthetic step is common to all ergot alkaloids , and after a series of further enzymatic steps through methyltransferases and oxygenases , the ergoline lysergic acid is formed. Lysergic acid is used as substrate by the enzyme lysergyl peptide synthetase in the subsequent steps and is successively bound to L -alanine, L- proline and L- phenylalanine. Enzymatic or spontaneous ring closure, oxygenation and epimerization , which take place in several steps , ultimately result in ergotamine as the end product.
Legal
Since ergotamine is used as a raw material for the synthesis of the forbidden psychedelic LSD , the supply is practically worldwide restricted by the respective raw material monitoring laws .
history
Ergotamine was first isolated in crystalline form at the Sandoz company under the direction of Arthur Stoll at the beginning of the 20th century and marketed as "Gynergen".
Trade names
- Monopreparations
- Ergo-Kranit migraine tablets (D)
- Gynergen (historical)
- Combination preparations
- Avamigran (A)
- Cafergot (D, CH)
- Secocapton (A)
- Synkapton (A)
Web links
- Swiss Medicines Compendium: Ergotamine Preparations
Individual evidence
- ↑ a b c d e f g h The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 627, ISBN 978-0-911910-00-1 .
- ↑ a b Ergotamine D-tartrate data sheet from Sigma-Aldrich , accessed on March 30, 2011 ( PDF ).
- ↑ ERGOTAMINE TARTRATE CRS data sheet (PDF) at EDQM , accessed on December 19, 2008.
- ↑ Hans-Christoph Diener (editor): Guidelines for diagnostics and therapy in neurology. Cluster headache and trigeminal autonomic headache. Pp. 567-572. Published by the "Guidelines" commission of the German Society for Neurology. Georg Thieme Verlag, 4th revised. Edition 2008; ISBN 3-13-132414-7 Online version of the DGN, 2015 .
- ↑ Schardl CL, Panaccione DG, Tudzynski P: ergot alkaloids - biology and molecular biology . In: Alkaloids Chem. Biol. Vol. 63, 2006, pp. 45-86 , doi : 10.1016 / S1099-4831 (06) 63002-2 , PMID 17133714 .
- ^ Alfons Metzner: Weltproblem Gesundheit , Imhausen International Company mbh , Lahr (Black Forest) 1961, p. 90.
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) , Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10 -9 , p. 180.